Lowering Detection Limits for 1,4-Dioxane in Drinking Water Using Large Volume Injection in an Unmodified Splitless GC Inlet
Abstract
Concurrent solvent recondensation–large volume splitless injection (CSR-LVSI) for gas chromatography typically requires a special injection port such as a programmable temperature vaporizer (PTV). The technique described here for the analysis of 1,4-dioxane in drinking water uses an unmodified split/splitless inlet with CSR-LVSI to lower reporting limits without concentrating the extract. This CSR-LVSI technique improves detectability with no specialized equipment or changes to standard laboratory instrumentation.
Introduction
Global concern over the carcinogenic potential of 1,4-dioxane, along with its identification as a Group 2B compound by the World Health Organization’s International Agency for Research on Cancer (IARC), has led to increased regulatory interest in this compound. For example, as a part of Unregulated Contaminant Monitoring Rule 3 (UCMR3), the U.S. EPA is requiring that all municipalities serving drinking water to more than 10,000 people monitor 1,4-dioxane levels for 12 consecutive months between 2013 and 2015. The mandated method for 1,4-dioxane analysis is EPA 522, which was developed for part-per-trillion (ppt) analysis of drinking waters using solid phase extraction (SPE) cartridges and gas chromatography-mass spectrometry (GC-MS) in selected ion monitoring (SIM) mode. 1,4-Dioxane is a synthetic organic solvent used to stabilize chlorinated solvents; 1,1,1-trichloroethane (TCA), for example, may contain up to 8% 1,4-dioxane. 1,4-Dioxane is very soluble in water, and improper disposal of chlorinated solvents can lead to the accumulation of 1,4-dioxane in ground and surface waters used as drinking water sources [1].
The 1x10-6 cancer risk assessment level for 1,4-dioxane was recently revised down to 0.35 µg/L from 3.0 µg/L by the U.S. EPA. This risk level corresponds to the lifetime probability of one individual in an exposed population of one million developing cancer. As a result, the proposed minimum reporting level (MRL) for 1,4-dioxane as part of UCMR3 is 0.07 µg/L (70 ppt) [2]. Using large volume splitless injection in GC-MS is advantageous when trying to analyze trace-level contaminants in clean matrices like drinking water because greater levels of target compounds are introduced onto the analytical column resulting in better detectability. Generally, a special injection port such as a programmed temperature vaporization (PTV) injector is required for this type of injection [1]. With the PTV inlet temperature set near the boiling point of the solvent, the sample is introduced at a high split ratio and as the solvent evaporates the analytes of interest are concentrated in the inlet. After a predetermined time, the split valve is closed and the inlet temperature is increased to transfer the concentrated sample and remaining solvent onto the column. This solvent-venting, analyte-concentrating step requires a relatively large difference in boiling points between solvent and solute, more than 100 °C, in order to prevent loss of analytes of interest to the split vent [3]. This rules out using a PTV type injection port for Method 522 due to inadequate differences in boiling point. The SPE elution solvent described in the method is dichloromethane (DCM), which has a boiling point of 40 °C. The analysis uses deuterated tetrahydrofuran (THF-d8), which has a boiling point between 65 and 66 °C, as the internal standard, while 1,4-dioxane and its deuterated isotopologue 1,4-dioxane-d8 both boil around 100 °C (101 and 99 °C respectively).
This lab has successfully demonstrated that concurrent solvent recondensation–large volume splitless injection (CSR-LVSI), a technique described by Magni and Porzano [4,5], can be used without any modification to an Agilent-style splitless injection port for a variety of analyses, including polycyclic aromatic hydrocarbons (PAHs), total petroleum hydrocarbons (TPH), EPA Method 8270 semivolatiles [6], brominated flame retardants [7], as well as many organochlorine, organonitrogen, and organophosphorus pesticides. The CSR-LVSI technique we used is based on the work of application chemists at Thermo Scientific, who have successfully applied their CSR-LVSI technique to drugs-of-abuse, pesticides, and polychlorinated biphenyls by injecting 20–35 µL, significantly improving limits of detection [8, 9, 10]. This technique uses a retention gap (e.g., 5 m x 0.25 mm) press-fitted to the analytical column and a starting GC oven temperature below the boiling point of the solvent. A fast autosampler injection with liquid band formation of the injected sample into a liner containing glass wool eliminates backflash in the hot injection port [11].
Given how close the boiling points of the compounds in question are to the cartridge elution solvent, we theorized that CSR-LVSI would be especially applicable for analyzing 1,4-dioxane in drinking water according to U.S. EPA Method 522. The typical procedure for preparing samples using SPE cartridges involves extracting a given volume of sample, drying the extract, and concentrating it down to a final volume of 1 mL. However, due to the high volatility of 1,4-dioxane and THF-d8, this concentration step is expressly forbidden in EPA Method 522 [12] in order to prevent evaporative loss of the target analytes. The authors of EPA Method 522 saw losses of 1,4-dioxane of over 30% when concentrating extracts using gentle nitrogen stream evaporation (N-Evap) and an ambient temperature water bath [1]. Our work assessed the potential for CSR-LVSI to lower detection limits, while not compromising 1,4-dioxane recoveries.
Experimental
To determine if CSR-LVSI was both compatible with the volatile compounds in question and an appropriate technique to lower detection limits, a 9-point calibration curve covering 3 orders of magnitude was prepared using 1,4-dioxane (cat.# 30287) (Table I). The surrogate standard, 1,4-dioxane-d8 (cat.# 30614), and the internal standard, THF-d8 (cat.# 30112), were both added at a concentration of 50 pg/µL.
Table I: Calibration curve (1-1,000 pg/µL).
| Level | Prepared Standard (pg/µL) | 10 µL Injection Amount On-Column (pg) |
Equivalent Concentration in 500 mL Samples (µg/L) |
|---|---|---|---|
| 1 | 1.0 | 10 | 0.020 |
| 2 | 2.0 | 20 | 0.040 |
| 3 | 10 | 100 | 0.20 |
| 4 | 50 | 500 | 1.0 |
| 5 | 100 | 1,000 | 2.0 |
| 6 | 250 | 2,500 | 5.0 |
| 7 | 500 | 5,000 | 10 |
| 8 | 750 | 7,500 | 15 |
| 9 | 1,000 | 10,000 | 20 |
The low point in the calibration is one-half the low point published for the MS SIM instrument in the 522 method, effectively halving the theoretical minimum detection limit. A second 5-point calibration curve covering 2 orders of magnitude was prepared with lower concentrations of internal standard and surrogate standard (0.010 µg/L vs. 0.050 µg/L) to probe the limits of the GC-MS system being used for the experiment and to attempt to halve the minimum detection limit again. Calibration levels and equivalent concentrations for each curve are shown in Tables I and II.
Table II: Calibration curve (0.5-50 pg/µL).
| Level | Prepared Standard (pg/µL) | 10 µL Injection Amount On-Column (pg) |
Equivalent Concentration in 500 mL Samples (µg/L) |
|---|---|---|---|
| 1 | 0.50 | 5.0 | 0.010 |
| 2 | 1.0 | 10 | 0.020 |
| 3 | 5.0 | 50 | 0.10 |
| 4 | 10 | 100 | 0.20 |
| 5 | 50 | 500 | 1.0 |
Solid Phase Extraction (SPE)
A thorough evaluation of the method-specific Resprep SPE cartridge for EPA 522 (cat.# 26032), which is a 6 mL cartridge with 2 g activated charcoal, is described in Grimmett and Munch's paper on the development of EPA Method 522 [1]. The cartridge is meant for samples ranging between 0.5 L and 1 L, though high levels of suspended solids may severely restrict flow or completely clog the cartridge before the full sample amount has passed. Average recoveries of 1,4-dioxane were in the mid 90 to low 100 percentile with %RSDs less than 5 (n=7 for each matrix) when the activated carbon SPE cartridges were used to extract 3 different drinking water sources (surface water, high total organic carbon surface water, and high mineral content ground water).
Our concern regarding the SPE cartridge is not extraction efficiency, but the consequences of injecting 10 times the normal amount of sample matrix into the GC. While operating in selected ion mode, especially when dealing with low molecular weight ions, it is critical that the peaks of interest be separated from interferences. The method explicitly states that the analyst must verify the absence of interferences for 1,4-dioxane at both the quantitation ion (m/z 88) and the confirmation ion (m/z 58). To evaluate the effects of the extract matrix on the analysis, several fortified samples and extraction blanks were prepared using deionized reagent water and bottled drinking water purchased from a local grocery store. The surrogate standard was added at 0.010 µg/L and 0.020 µg/L, depending on the amount of sample extracted, so that the extract would have a final surrogate concentration of 0.010 µg/mL. The bottled waters were fortified while still in their plastic bottles, recapped, mixed by inversion and allowed to sit for several hours to ensure homogeneous samples.
Each sample was extracted using a Resprep activated coconut charcoal SPE cartridge following EPA Method 522 protocol. Immediately after solvent elution, the extracts were spiked with 20 µL of internal standard and brought up to 10 mL final volume as specified in the method, resulting in a concentration of 10 pg/µL in the extract. The extracts were then transferred to a large storage vial and dried with anhydrous magnesium sulfate to take advantage of its more complete drying capacity (this was a deviation from the method, which calls for sodium sulfate).
GC-MS Conditions
A fast autosampler injection with an Agilent 7683 injector was used to make large volume (10 µL) injections from a 25 µL SGE large volume autosampler syringe (cat.# 24798). A Restek Premium 4 mm single taper liner with wool (cat.# 23303) placed at the bottom of the liner was installed into an unmodified Siltek treated EZ Twist Top split/splitless injection port at 120 °C. The purge valve time was set at 1 minute. A deactivated 5 m x 0.25 mm ID Rxi retention gap (cat.# 10029) was installed in the injector and press-fitted (deactivated Universal Press-Tight connector, cat.# 20429) to a 30 m x 0.25 mm x 1.4 µm Rxi-624Sil MS column (cat.# 13868). The oven was programmed from 35 °C (hold 1 min) to 120 °C (hold 1 min) at 12 °C/min, with a constant flow of 1.4 mL/min helium carrier gas. All analyses were performed on an Agilent 7890A GC with a 5975C quadrupole mass spectrometer operated in selected ion monitoring mode.
Results and Discussion
The goal of this work was to assess the potential for using CSR-LVSI with a completely unmodified standard split/splitless GC inlet as a means of lowering detection limits for 1,4-dioxane in drinking water. To first determine if the technique was appropriate for this application, peak response, linearity, and interferences were assessed.
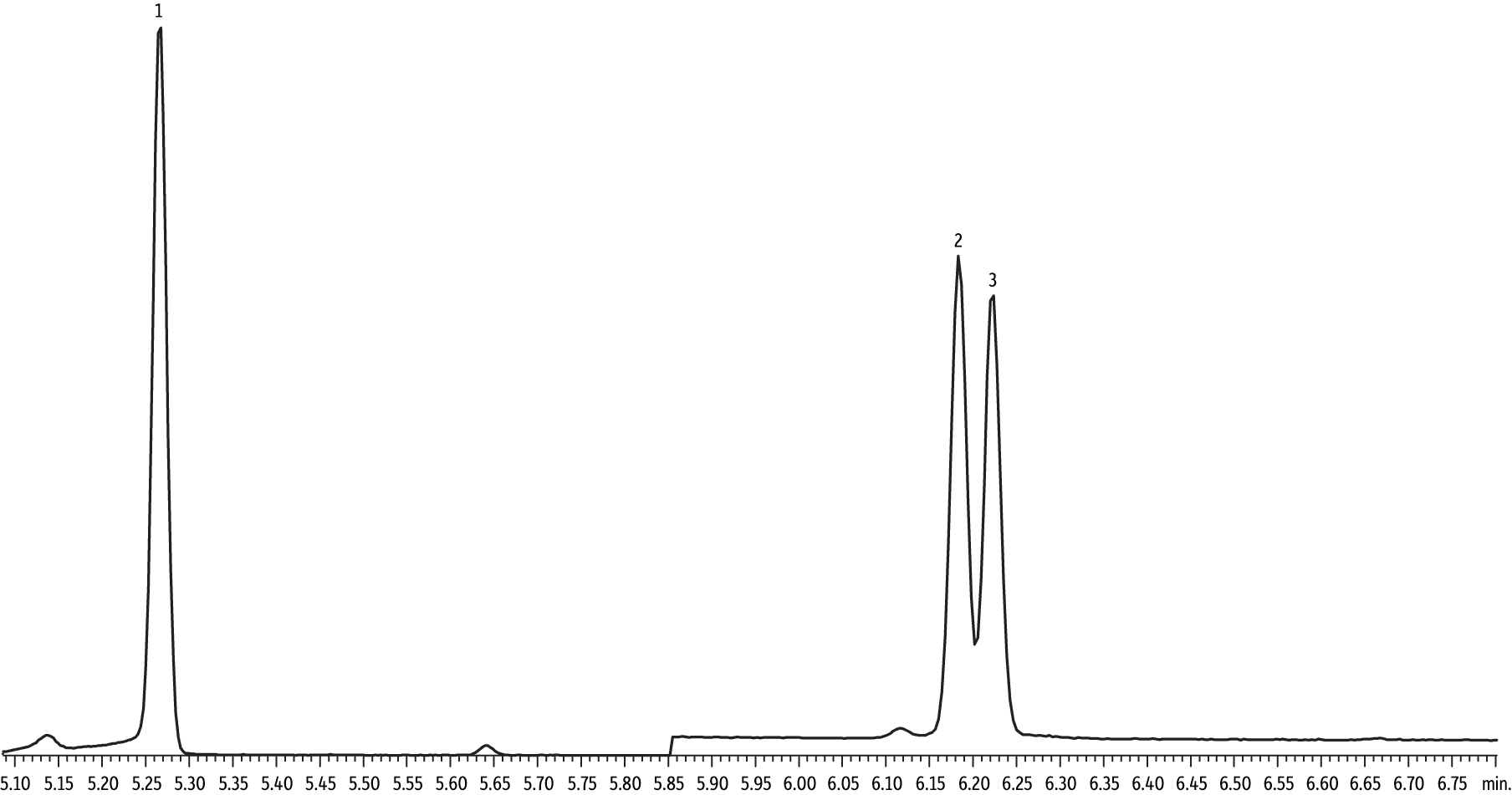
With large volume injections, there can be concern about analyte loss; however, this would be apparent in a comparison of CSR-LVSI and standard volume injections that delivered equivalent on-column amounts. If no sample loss occurs, the peak area obtained using the CSR-LVSI technique should correspond with the peak area of standard volume splitless injection. Figure 1 is an overlay of extracted ion chromatograms for the 1,4-dioxane quantitation ion (m/z 88). The peak on the left is a 1 µL splitless injection of a 500 pg/µL standard. The peak on the right is a 10 µL splitless injection of a 50 pg/µL standard. Though retention time and peak shape vary greatly, the peak areas are comparable, indicating that no sample was lost when using the CSR-LVSI technique. Also, the use of CSR-LVSI improves peak shape, which is a phenomenon we have seen before [6]. The recondensation of the solvent and analytes in the cool oven and subsequent re-evaporation of the solvent (DCM) as the oven program passes 40 °C focuses the solutes into a very narrow band before they are separated by the analytical column, resulting in a narrow, symmetrical 1,4-dioxane peak.
Figure 1: Overlay of CSR-LVSI and standard volume injections. Comparable areas for equivalent on-column amounts from both injection techniques indicate no loss of sample occurred when using CSR-LVSI.
| Peaks | |
|---|---|
| 1. | 1 µL injection of 500 pg/µL 1,4-dioxane standard (500 pg on-column) |
| 2. | 10 µL injection of 50 pg/µL 1,4-dioxane standard (500 pg on-column) |
| Column | Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|---|---|
| using Rxi guard column 5 m, 0.25 mm ID (cat.# 10029) | |
| with Universal Press-Tight connectors (cat.# 20429) | |
| Standard/Sample | 1,4-Dioxane (cat.# 30287) |
| Diluent: | Dichloromethane |
| Injection | splitless (hold 1 min) |
| Liner: | Premium 4 mm single taper w/wool (cat.# 23303) |
| Inj. Temp.: | 120 °C |
| Purge Flow: | 80 mL/min |
| Oven | |
| Oven Temp.: | 35 °C (hold 1 min) to 120 °C at 12 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Linear Velocity: | 30.556 cm/sec @ 35 °C |
| Detector | MS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode: | SIM | ||||||||||||
| SIM Program: | |||||||||||||
| |||||||||||||
| Transfer Line Temp.: | 280 °C | ||||||||||||
| Analyzer Type: | Quadrupole | ||||||||||||
| Source Temp.: | 230 °C | ||||||||||||
| Quad Temp.: | 150 °C | ||||||||||||
| Solvent Delay Time: | 5.0 min | ||||||||||||
| Tune Type: | BFB | ||||||||||||
| Ionization Mode: | EI | ||||||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||||||
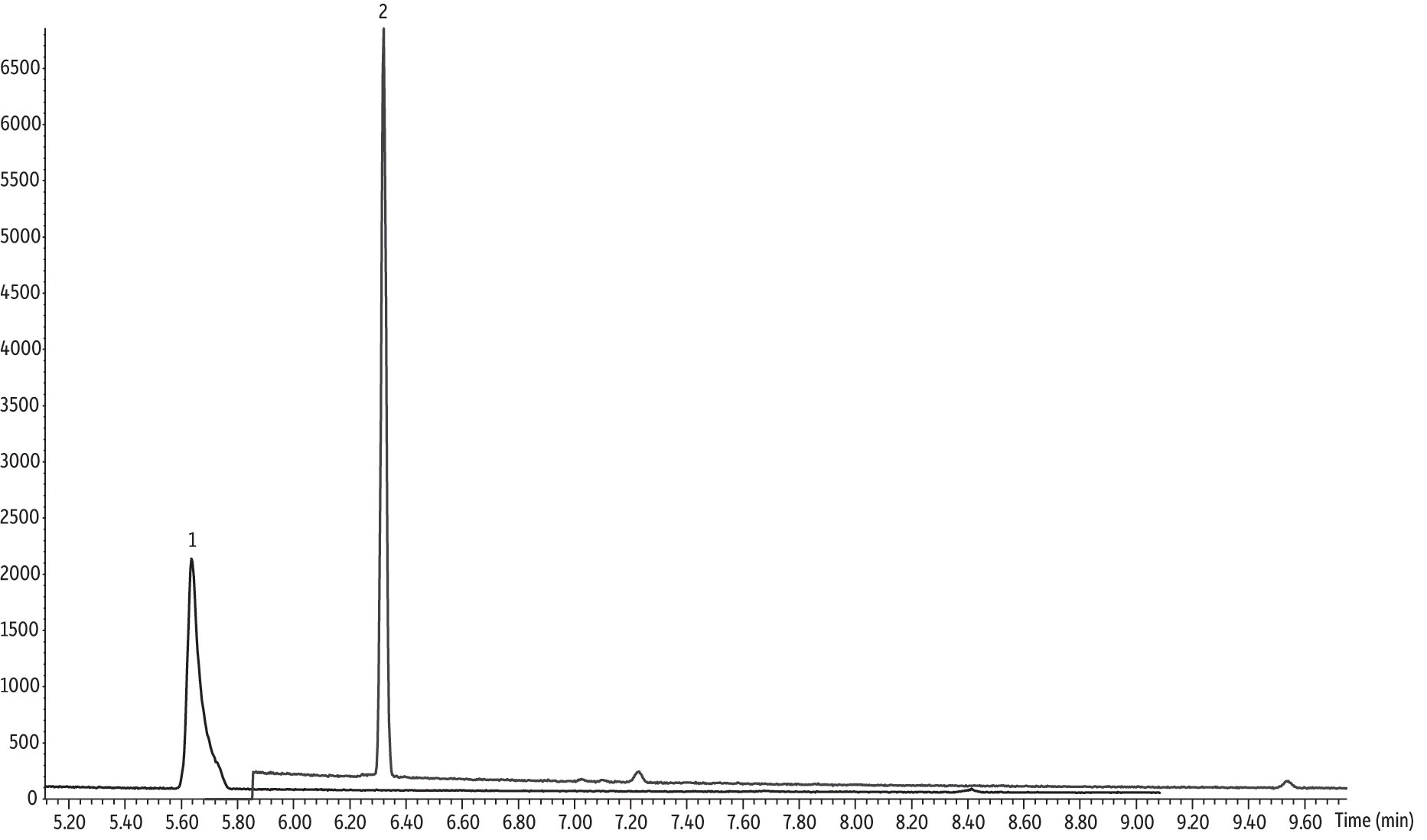
Another concern when using large injection volumes in splitless injection is analyte loss due to backflash. Backflash is thought to occur when the solvent vapor expands to the point that its volume exceeds the capacity of the inlet liner, causing carryover, poor reproducibility, nonlinear increases in peak area with increasing injection volume, and loss of analytes via the septum purge valve. 1,4-Dioxane and THF have boiling points close enough to that of dichloromethane that if backflash were to occur a significant amount of these analytes could be lost to the explosive expansion of the solvent. Magni and Porzano suggest that backflash is prevented in CSR-LVSI by a pressure surge triggered by the rapid expansion of solvent after a fast CSR-LVSI into a hot GC inlet [4]. This sudden surge in pressure causes a much faster transfer of solvent and sample onto the column, while at the same time preventing the backflow of sample out the septum purge. For performing CSR-LVSI in our unmodified inlet, we chose an injection port temperature of 120 °C and none of the deleterious effects commonly associated with backflash were observed. Sample loss due to backflash would have manifested as nonlinear area responses, but excellent linearity was achieved across a wide calibration range that extended well below the EPA Method 522 minimum detection limit (Figures 2 and 3). A typical chromatogram for a 50 pg/µL standard is shown for reference in Figure 4.
Figure 2: 1 to 1000 pg/µL 1,4-dioxane calibration curve using 10 µL CSR-LVSI GC-MS.
Figure 3: 0.5 to 50 pg/µL 1,4-dioxane calibration curve using 10 µL CSR-LVSI GC-MS.
Figure 4: Analysis of a 10 µL 50 pg/µL 1,4-dioxane standard on an Rxi-624Sil MS column using CSR-LVSI in an unmodified split/splitless GC inlet.
| Peaks | tR (min) | |
|---|---|---|
| 1. | Tetrahydrofuran-D8 | 5.27 |
| 2. | 1,4-Dioxane-d8 | 6.19 |
| 3. | 1,4-Dioxane | 6.23 |
| Column | Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|---|---|
| using Rxi guard column 5 m, 0.25 mm ID (cat.# 10029) | |
| with universal angled Press-Tight connectors | |
| Standard/Sample | Tetrahydrofuran-d8 (cat.# 30112) |
| 1,4-Dioxane-d8 (cat.# 30614) | |
| 1,4-Dioxane (cat.# 30287) | |
| Diluent: | Dichloromethane |
| Conc.: | 50 ng/mL |
| Injection | |
| Inj. Vol.: | 10 µL splitless (hold 1 min) |
| Liner: | Premium 4 mm single taper w/wool (cat.# 23303) |
| Inj. Temp.: | 120 °C |
| Purge Flow: | 80 mL/min |
| Oven | |
| Oven Temp.: | 35 °C (hold 1 min) to 120 °C at 12 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Linear Velocity: | 30.556 cm/sec @ 35 °C |
| Detector | MS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode: | SIM | ||||||||||||
| SIM Program: | |||||||||||||
| |||||||||||||
| Transfer Line Temp.: | 280 °C | ||||||||||||
| Analyzer Type: | Quadrupole | ||||||||||||
| Source Temp.: | 230 °C | ||||||||||||
| Quad Temp.: | 150 °C | ||||||||||||
| Solvent Delay Time: | 5.0 min | ||||||||||||
| Tune Type: | BFB | ||||||||||||
| Ionization Mode: | EI | ||||||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||||||
| Notes | Connector cat.# 20446-261 was used to produce this chromatogram, but has since been discontinued. For assistance choosing a replacement for this application, contact Restek Technical Service or your local Restek representative. - - - - - - Large volume splitless injection for EPA Method 522 without a PTV. | ||||||||||||
| Acknowledgement | M. Misselwitz, J. Cochran | ||||||||||||
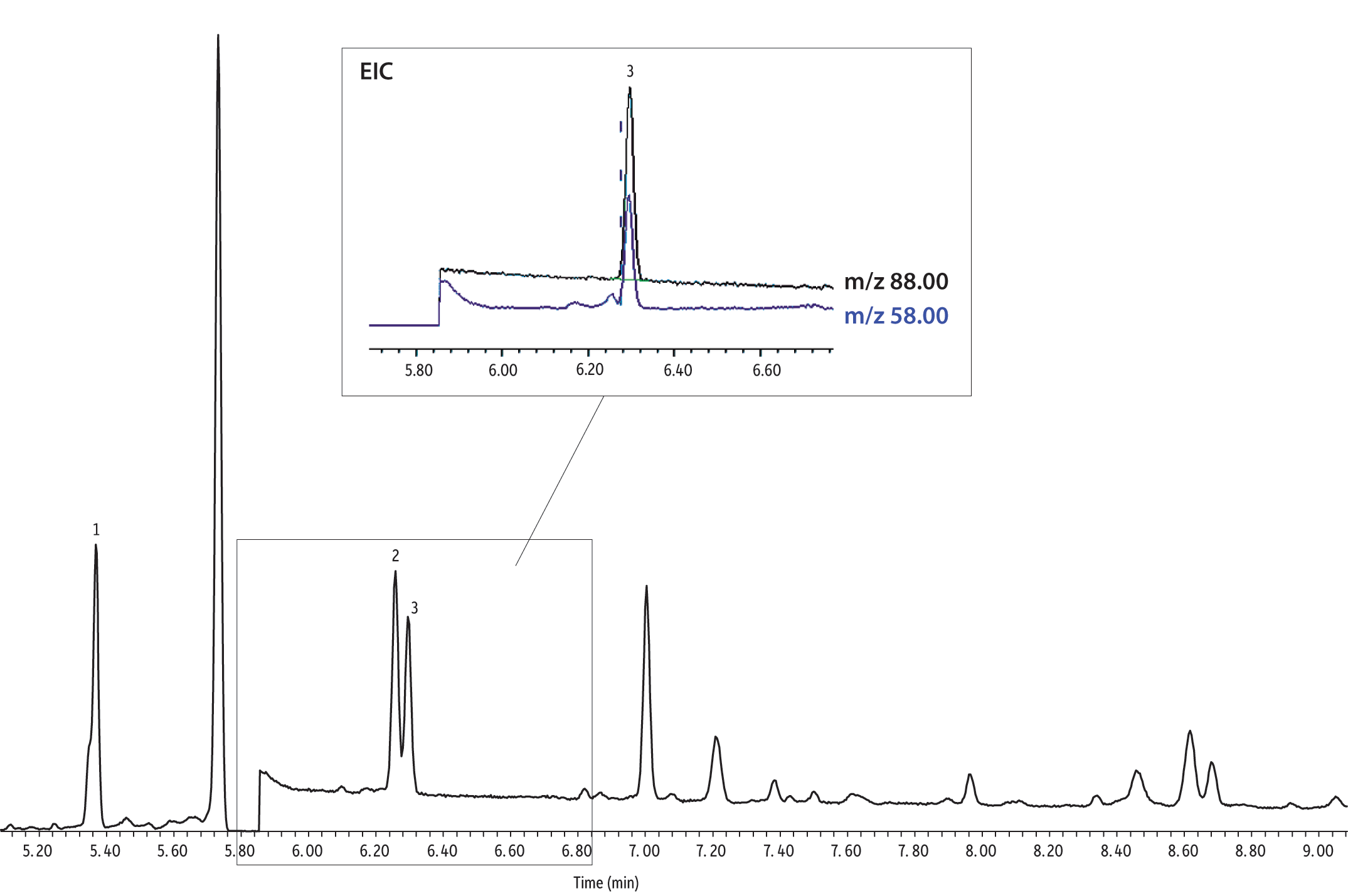
Peak response and linearity evaluations indicate that CSR-LVSI with an unmodified inlet results in complete transfer of 1,4-dioxane to the analytical column, with the additional benefit of improved peak shape. While results for injected standards were quite promising, large volume injections of sample extracts can introduce a significant amount of co-extracted material and the impact of potential interferences must be considered when assessing the technique. This analysis is especially sensitive to interference from co-extracted material because the SIM ions are at a relatively low mass to charge ratio. The extracted ion chromatogram for an unfortified reagent water extract is shown in Figure 5; note that the 1,4-dioxane-d8 surrogate (5.95 min, m/z 96 and 64) and the THF-d8 internal standard (5.1 min, m/z 46 and 78) are at 10 pg/µL. While some peaks from co-extracted materials are present and an elevated baseline for m/z 88 is seen, the chromatogram is free of critical interferences for 1,4-dioxane, and this was consistent throughout the analysis of cartridge extracts. As shown in the analysis of a fortified drinking water extract in Figure 6, 1,4-dioxane is chromatographically separated from any interferences using the CSR-LVSI technique with analysis on an Rxi-624Sil MS column.
Figure 5: Extracted ion chromatograms for 10 µL CSR-LVSI GC-MS of an unfortified reagent water extract. Although the baseline is slightly elevated and peaks for some co-extracted material are present, no critical interferences for 1,4-dioxane (m/z 88) occur.
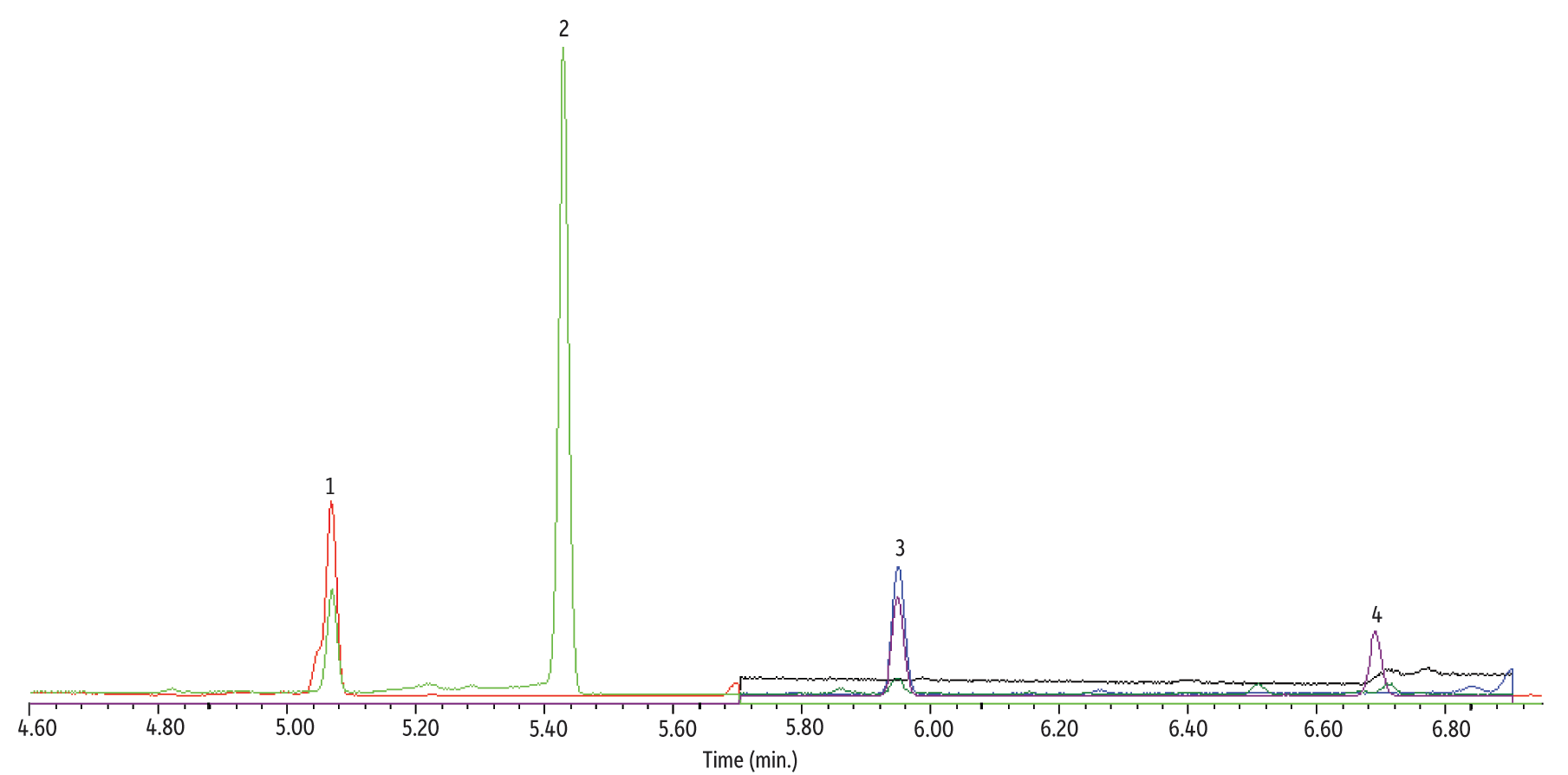
| Peaks | tR (min) | m/z | |
|---|---|---|---|
| 1. | Tetrahydrofuran-d8 (IS) | 5.1 | 46,78 |
| 2. | Co-extracted material | - | 78 |
| 3. | 1,4-Dioxane-d8 (SS) | 5.95 | 96,64 |
| 4. | Co-extracted material | - | 64 |
See Figure 4 for instrument conditions.
Figure 6: Extracted ion chromatogram of a 0.5 pg/µL fortified drinking water extract (10 µL CSR-LVSI, 5 pg on-column). Note that the 1,4-dioxane quantification ion (m/z 88) and confirmation ion (m/z 58) are fully separated from matrix interferences, as required by EPA Method 522.
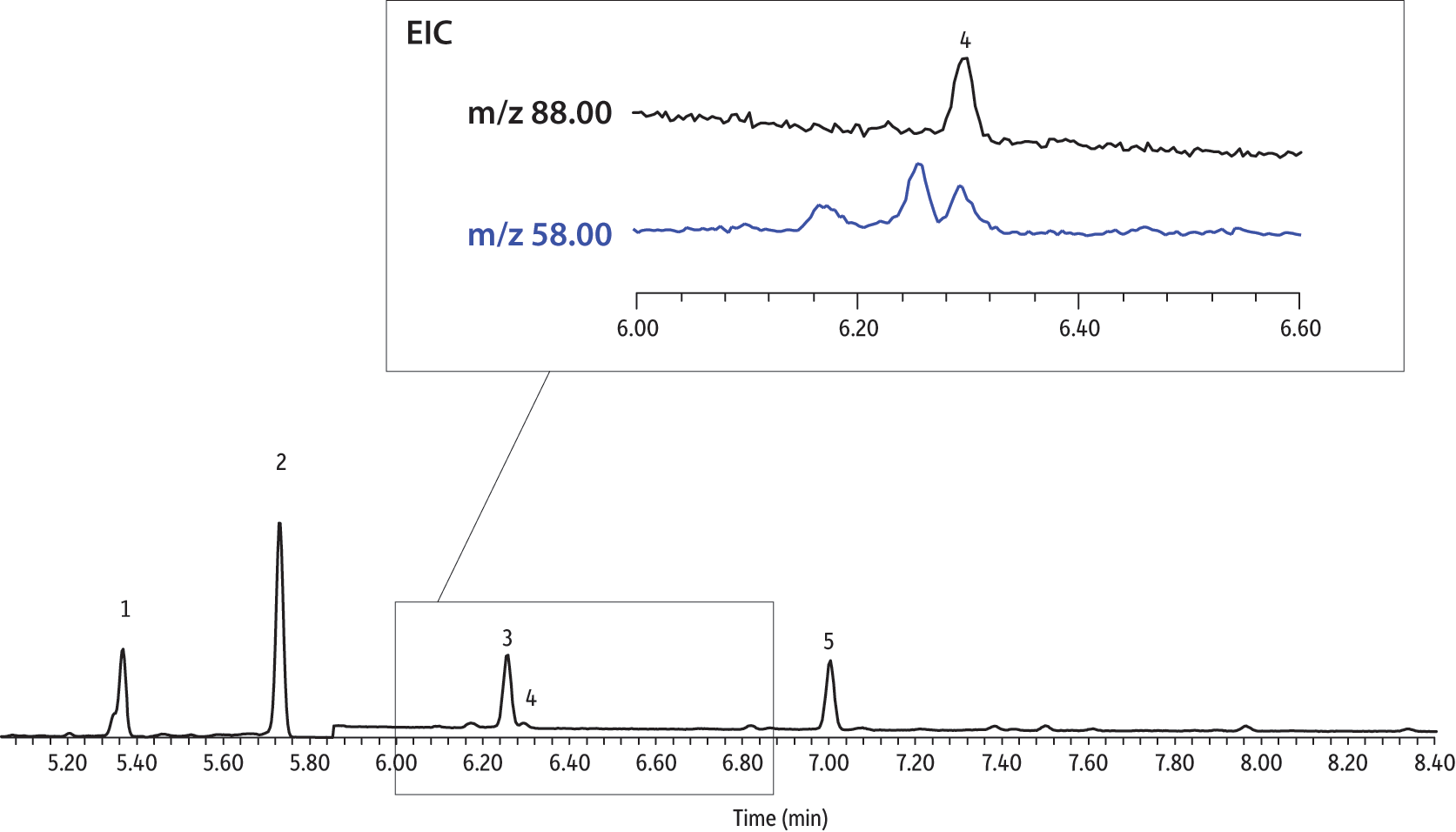
| Peaks | |
|---|---|
| 1. | Tetrahydrofuran-d8 (IS) |
| 2. | Co-extracted material |
| 3. | 1,4-Dioxane-d8 (SS) |
| 4. | 1,4-Dioxane |
| 5. | Co-extracted material |
See Figure 4 for instrument conditions.
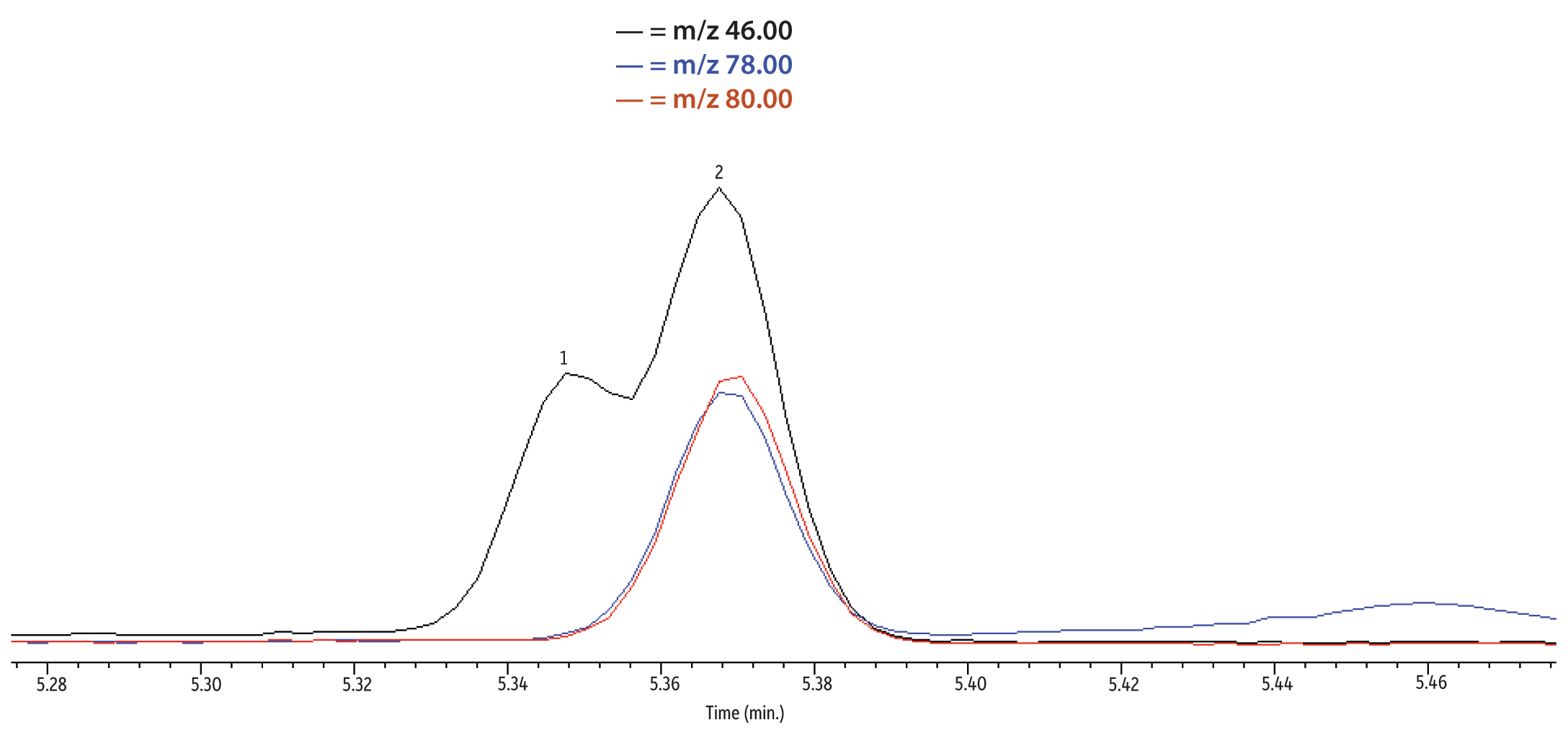
While we were able to separate 1,4-dioxane from any interferences in the deionized and drinking water extracts, there was a partial co-elution with m/z 46 (THF-d8 recommended quantitation ion) in the extracted samples that was not present in the analyzed standards (Figure 7). However, the suggested confirmation ions, m/z 78 and m/z 80, were both free of interferences in all extracted sample runs with good responses, making either ion an acceptable replacement for m/z 46.
Figure 7: Extracted ion chromatogram of an extracted drinking water sample. A partial coelution is seen for the THF-d8 internal standard quantitation ion (m/z 46), but both confirmation ions are free of interferences and could be used for quantification.
See Figure 4 for instrument conditions.
The CSR-LVSI with an unmodified inlet approach used here allowed a 10-fold increase in sample to be injected without significant interference from matrix components. However, the potential for interferences with large volume injection is always a concern and EPA Method 522 raises an important point about the contribution of carryover to peak interferences. Even though 1,4-dioxane elutes at 6.3 minutes, the oven program and run should continue until all matrix compounds have eluted off of the column. In the method, the author elutes 1,4-dioxane around 9 minutes on a 624-type column with a starting oven temperature of 30 °C, but then continues the run for another 9 minutes to completely elute the co-extracted materials. While most 624-type columns have maximum operating temperatures of 240 to 260 °C, the Rxi-624Sil MS column used here has an extended maximum operating temperature of 320 °C. This allows a higher temperature hold at the end of the analysis to remove residual compounds from the GC column, which reduces the potential for carryover of analytes that could interfere with the compounds of interest. The higher temperature limit of the Rxi-624Sil MS column can help analysts increase sample throughput, given that less volatile compounds will elute faster at 320 °C than at 260 °C. Additionally, increased throughput is possible since the starting oven temperature of 35 °C is more quickly obtained than the 30 °C oven start used in Method 522.
Having established that using CSR-LVSI with an unmodified GC inlet is an appropriate technique for analyzing 1,4-dioxane in drinking water, we wanted to further assess its potential for lowering detection limits in matrix. As previously shown in Figure 6, the extracted ion chromatogram of a CSR-LVSI of a bottled water sample extract (water fortified to 0.0050 µg/L) highlights the separation of the m/z 58 confirmation ion from a closely eluting matrix compound. At approximately 10 pg on-column, the measured signal-to-noise ratio for m/z 88 (quantitation ion) is 16, which is above the accepted threshold of 10. When 1 µL of the same extract is injected, the resulting 1,4-dioxane peak is barely distinguishable from the noise and the confirmation ion cannot be seen (Figure 8). For a sample fortified at 0.20 µg/L (10 pg/µL in extract), the difference between a 1 µL injection (Figure 9) and a 10 µL injection (Figure 10) is still striking. Ultimately, the improved signal-to-noise ratios obtained using CSR-LVSI resulted in recoveries of 1,4-dioxane and 1,4-dioxane-d8 that were within the expected range (Table III) and matched the published method development data very well [1].
Figure 8: 1,4-Dioxane extracted ion chromatogram of a 0.5 pg/µL extract (standard splitless 1 µL injection, 0.5 pg on-column).
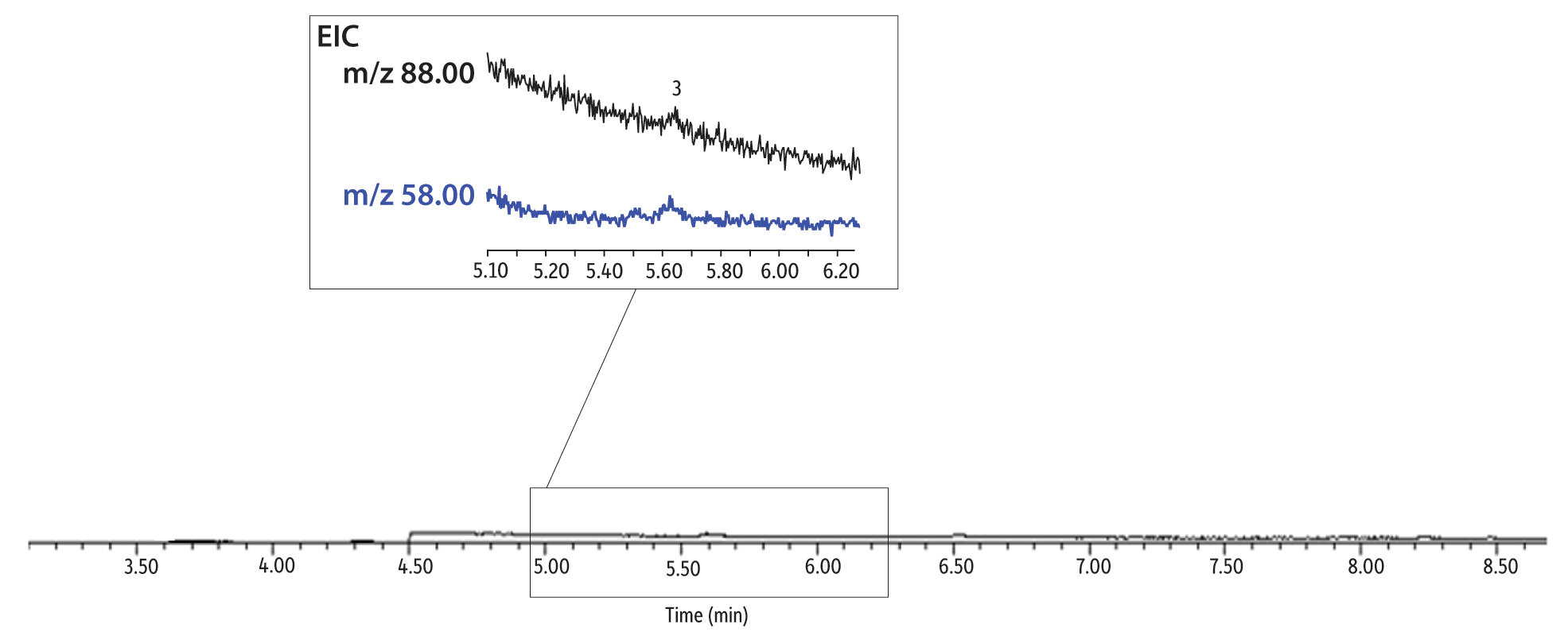
| Peaks | |
|---|---|
| 1. | Tetrahydrofuran-d8 (IS) |
| 2. | 1,4-Dioxane-d8 (SS) |
| 3. | 1,4-Dioxane |
See Figure 4 for instrument conditions.
Figure 9: 1,4-Dioxane extracted ion chromatogram of a 10 pg/µL extract (standard splitless 1 µL injection, 10 pg on-column).
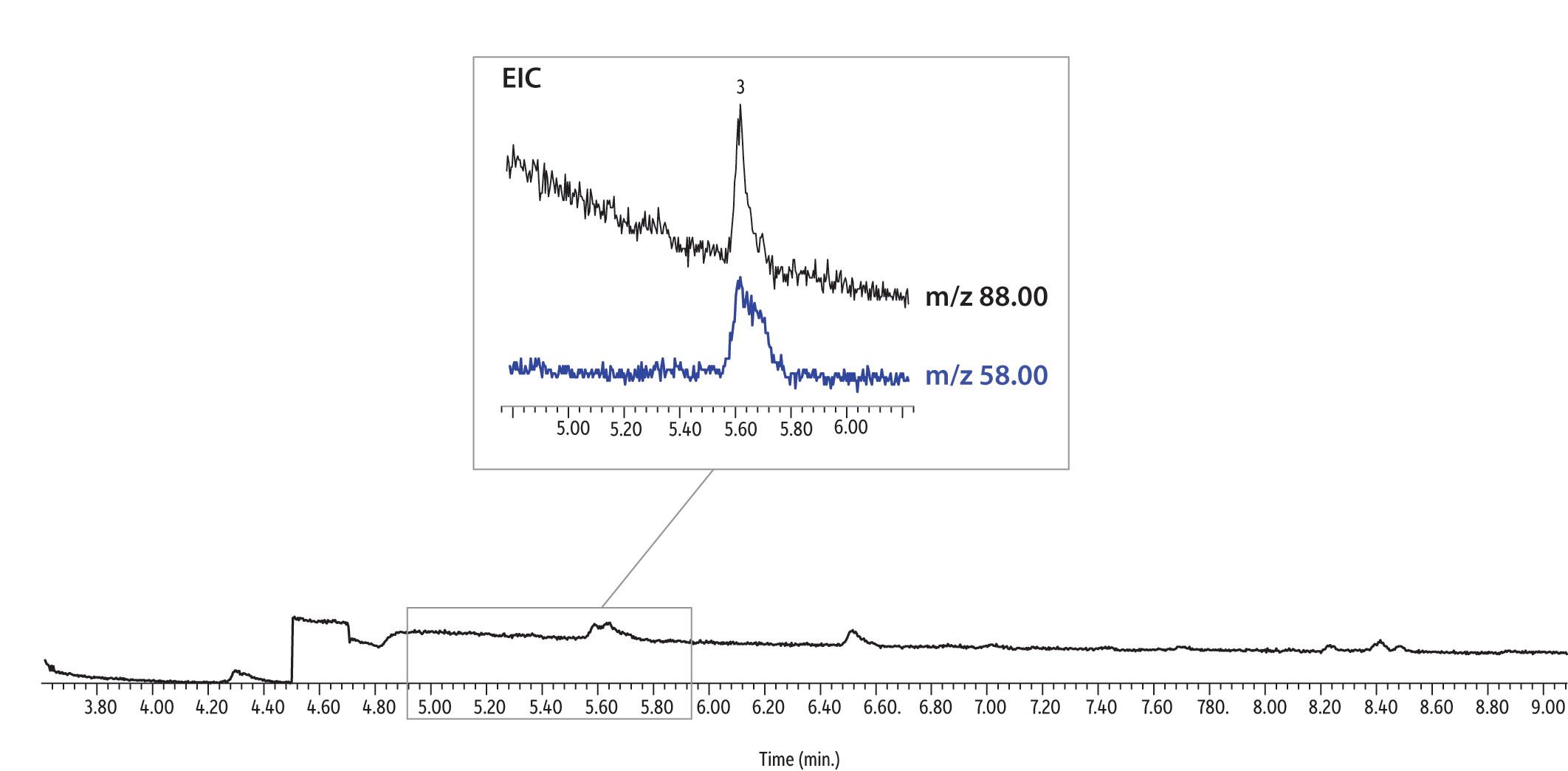
| Peaks | |
|---|---|
| 1. | Tetrahydrofuran-d8 (IS) |
| 2. | 1,4-Dioxane-d8 (SS) |
| 3. | 1,4-Dioxane |
See Figure 4 for instrument conditions.
Figure 10: 1,4-Dioxane extracted ion chromatogram of a 10 pg/µL extract (10 µL CSR-LVSI, 100 pg on-column).
See Figure 4 for instrument conditions.
Table III: CSR-LVSI resulted in good recovery of both 1,4-dioxane and surrogate 1,4-dioxane-d8 from extracted fortified samples.
| Matrix | Fortified Sample Conc. (µg/L) | Volume of Sample Extracted (L) | Theoretical Extract Conc. (pg/µL) | Recovery (pg/µL) | 1,4-Dioxane % Recovery |
Surrogate % Recovery |
| Bottled drinking water | 0.0050 | 1.0 | 0.50 | 0.40 | 80 | 125 |
| Bottled drinking water | 0.20 | 0.50 | 10 | 9.2 | 92 | 102 |
| Bottled drinking water | 0.20 | 1.0 | 20 | 18 | 87 | 96 |
| Reagent water | 0.020 | 0.50 | 1.0 | 1.0 | 100 | 88 |
| Reagent water | 0.20 | 0.50 | 10 | 8.4 | 84 | 92 |
| Reagent water | 0.0 | 0.50 | 0.0 | - | - | 86 |
Conclusion
Concurrent solvent recondensation–large volume splitless injection with an unmodified Agilent split/splitless GC inlet has been shown to be a technically viable approach with potential advantages for EPA Method 522 dioxane analysis. The solvent recondensation focuses the solutes into a tight band on the column, which improves chromatography. At the same time, this technique increases the amount of analyte transferred to the column. Using CSR-LVSI with a standard inlet, as shown here, provides a cost-effective way to meet ever decreasing detection and quantitation limits.