Analysis of Amphetamines by LC-MS/MS for High-Throughput Urine Drug Testing Labs
Abstract
Accurate analysis of amphetamines by LC-MS/MS can be achieved using chiral columns, but the analysis presented here provides a simpler, more cost-effective approach for high-throughput labs. Excellent separation of all enantiomers in urine was obtained in a fast, 7-min quantitative analysis on a Raptor C18 column with no interference from matrix components.
Introduction
Amphetamine and methamphetamine are psychostimulant drugs that occur as two enantiomers, dextrorotary and levorotary, as a result of their chiral center. The dextromethamphetamine (d-isomer) form is highly abused and typically found in illicit preparations. However, detection of abuse is complicated because consumption of over-the-counter and prescription medications may yield positive results if the analytical method used cannot distinguish between the d- and l- enantiomers. Chiral separation of d- and l-methamphetamine and their metabolites d- and l-amphetamine (Figure 1) can help determine whether the source was licit or illicit, however, chiral columns can be expensive, may necessitate a dedicated instrument, and are not as broadly useful as ubiquitous C18 columns.
In order to provide labs with a high-throughput assay that effectively separates amphetamines by LC-MS/MS in urine without the use of a costly and specialized chiral column, we developed the following analysis on a standard reversed-phase Raptor C18 column. The method employs a simple precolumn derivatization followed by dilution and results in a selective, specific analysis of d- and l-amphetamine and methamphetamine enantiomers in urine that is free from sample matrix interferences.
Figure 1: Structures of d- and l-Amphetamine and Methamphetamine Enantiomers.
Experimental
Calibration Standards and Quality Control Samples
Analyte-free pooled human urine (BioIVT) was fortified with d- and l-amphetamines and d- and l-methamphetamines (Cerilliant) to prepare seven calibration standards and four QC samples. The linearity range was 50–5000 ng/mL. Four QC levels were prepared at 50, 125, 700, and 4000 ng/mL. The fortified calibration standards and QC samples were subjected to the following sample preparation procedure.
Sample Preparation
50 μL of calibration standard or QC sample was aliquoted into a microcentrifuge tube. 10 μL of a working internal standard (20 μg/mL (±)-amphetamine-D11 and (±)-methamphetamine-D11 in water) and 20 μL of 1M NaHCO₃ was added and vortexed at 3000 rpm for 10 seconds. After vortexing, 100 μL of 0.1% (w/v) Marfey’s reagent (1-fluoro-2-4-dinitrophenyl-5-L-alanine amide) prepared in acetone was added, vortexed, and heated at 45 °C for 1 hour. Samples were allowed to cool to room temperature before the addition of 40 μL of 1M HCl in water. The sample was then vortexed and evaporated to dryness under nitrogen at 45 °C. Samples were reconstituted in 1 mL of 40:60 water:methanol (v/v) and filtered using Thomson SINGLE StEP standard filter vials (cat.# 28307) and then injected.
Optimization of Derivatization Procedure
To obtain the best sensitivity and to achieve 100% derivatization, a series of experiments were performed using a 4000 ng/mL d- and l-amphetamines and d- and l-methamphetamines sample prepared in water. To determine the optimal derivatization conditions, various incubation times and volumes were assessed. For the incubation time experiment, samples were incubated at 45 °C with 100 µL 0.1% (w/v) Marfey's reagent for 15, 30, 45, 60, or 90 minutes. For the volume experiment, varying volumes of 0.1% w/v Marfey's reagent (25, 50, 100, or 200 µL) were added to the samples, followed by incubation at 45 °C for 60 minutes. All the samples were subjected to the sample preparation procedure previously described and all conditions were evaluated in quadruplicate.
Instrument Conditions
Analysis of amphetamines by LC-MS/MS was performed on a Shimadzu Prominence HPLC equipped with a SCIEX API 4000 MS/MS. Instrument conditions were as follows and analyte transitions are provided in Table I.
| Analytical column: | Raptor C18 2.7 µm, 100 mm x 2.1 mm (cat.# 9304A12) | |
| Guard column: | Raptor C18 EXP guard column cartridge (cat.# 9304A0252) | |
| Mobile phase A: | 0.1% Formic acid in water | |
| Mobile phase B: | 0.1% Formic acid in methanol | |
| Gradient | Time (min) | %B |
| 0.00 | 60 | |
| 5.00 | 60 | |
| 5.01 | 90 | |
| 5.50 | 90 | |
| 5.51 | 60 | |
| 7.00 | 60 | |
| Flow rate: | 0.5 mL/min | |
| Injection volume: | 10 µL | |
| Column temp.: | 35 °C | |
| Ion mode: | Negative ESI | |
|
Compound |
Retention Time (min) |
Precursor Ion |
Product Ion Quantifier |
Product Ion Qualifier |
|---|---|---|---|---|
|
l-MAMP-D11 |
2.98 |
411.2 |
350.2 |
335.3 |
|
l-MAMP |
3.11 |
400.3 |
339.0 |
323.8 |
|
d-MAMP-D11 |
3.24 |
411.2 |
350.2 |
335.3 |
|
d-MAMP |
3.38 |
400.3 |
339.0 |
323.8 |
|
l-AMP-D11 |
3.97 |
397.2 |
336.0 |
319.0 |
|
l-AMP |
4.16 |
386.1 |
325.0 |
308.0 |
|
d-AMP-D11 |
4.34 |
397.2 |
336.0 |
319.0 |
|
d-AMP |
4.55 |
386.1 |
325.0 |
308.0 |
Results and Discussion
Optimization of Derivatization Procedure
An initial set of experiments was conducted to determine which reagent volumes and incubation times produced the most complete derivatization and best sensitivity. As shown in Figure 2, the highest peak responses were obtained using 100 µL of 0.1% (w/v) Marfey’s reagent with a 60-minute incubation time at 45 °C. These optimized derivatization conditions were used for all subsequent experiments.
Figure 2: Peak response and sensitivity were maximized using an optimized derivatization procedure (100 µL of 0.1% (w/v) Marfey’s reagent and a 60-minute incubation at 45 °C).
Effect of Incubation Time (100 µL of 0.1% Marfey's Reagent)
Effect of Derivatization Reagent Volume
Chromatographic Performance
This analysis of amphetamines by LC-MS/MS produced excellent separations and quantitative results in 7 minutes (Figure 3). Using a simple derivatization and dilution procedure—along with a Raptor C18 column—good baseline resolution of the target compounds was obtained, allowing easy peak identification and quantitation. Reproducible chromatographic performance (retention, peak shape, and sensitivity) was achieved over the course of 500 continuous injections, demonstrating good method robustness. Carryover was not observed.
Figure 3: Good chromatographic separation allows easy identification and quantitation in a 7-min analysis (500 ng/mL fortified human urine).
TIC
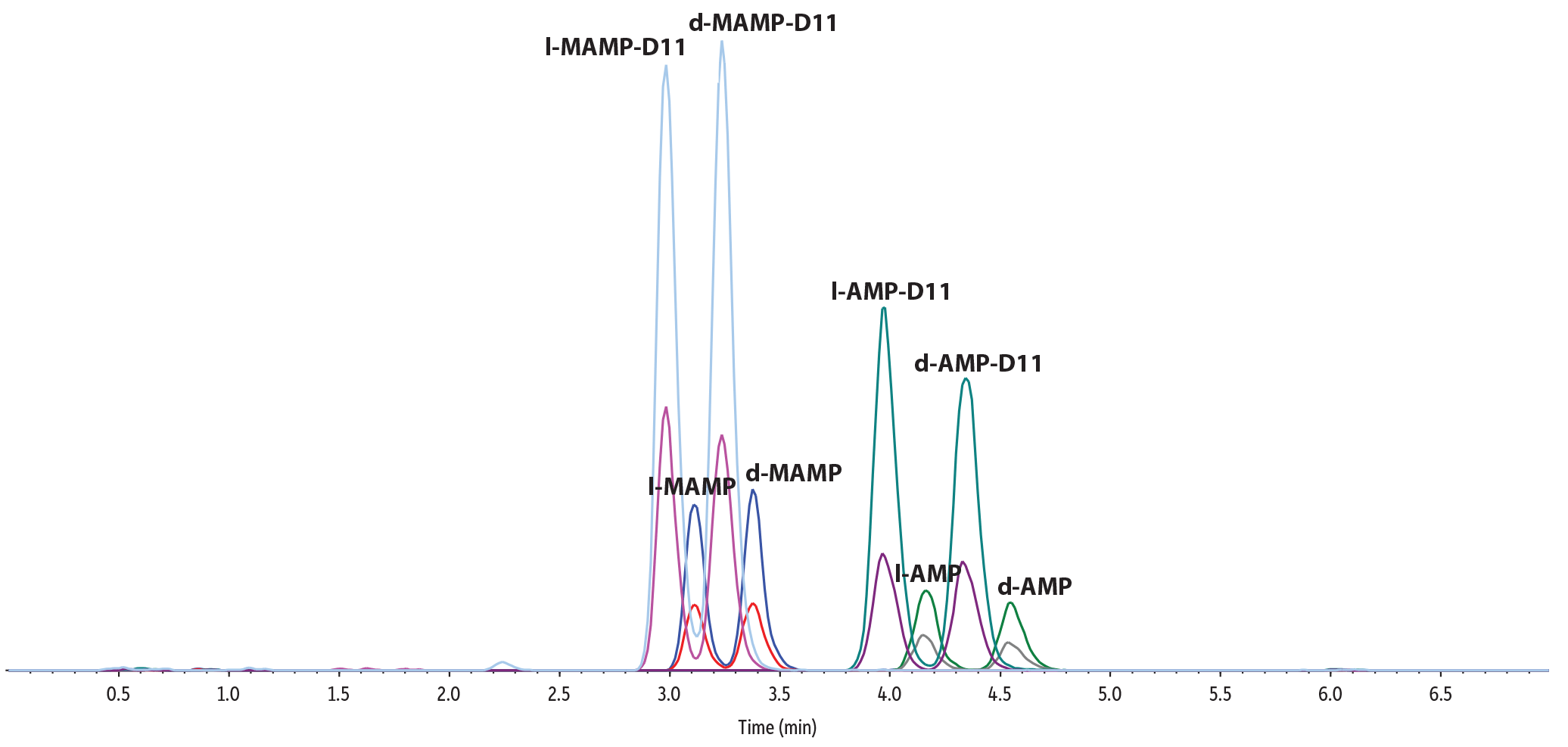
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | l-Methamphetamine-D11 (l-MAMP-D11) | 2.98 | 200 | 411.2 | 350.2 | 335.3 |
| 2. | l-Methamphetamine (l-MAMP) | 3.11 | 500 | 400.3 | 339.0 | 323.8 |
| 3. | d-Methamphetamine-D11 (d-MAMP-D11) | 3.24 | 200 | 411.2 | 350.2 | 335.3 |
| 4. | d-Methamphetamine (d-MAMP) | 3.38 | 500 | 400.3 | 339.0 | 323.8 |
| 5. | l-Amphetamine-D11 (l-AMP-D11) | 3.97 | 200 | 397.2 | 336.0 | 319.0 |
| 6. | l-Amphetamine (l-AMP) | 4.16 | 500 | 386.1 | 325.0 | 308.0 |
| 7. | d-Amphetamine-D11 (d-AMP-D11) | 4.34 | 200 | 397.2 | 336.0 | 319.0 |
| 8. | d-Amphetamine (d-AMP) | 4.55 | 500 | 386.1 | 325.0 | 308.0 |
XIC
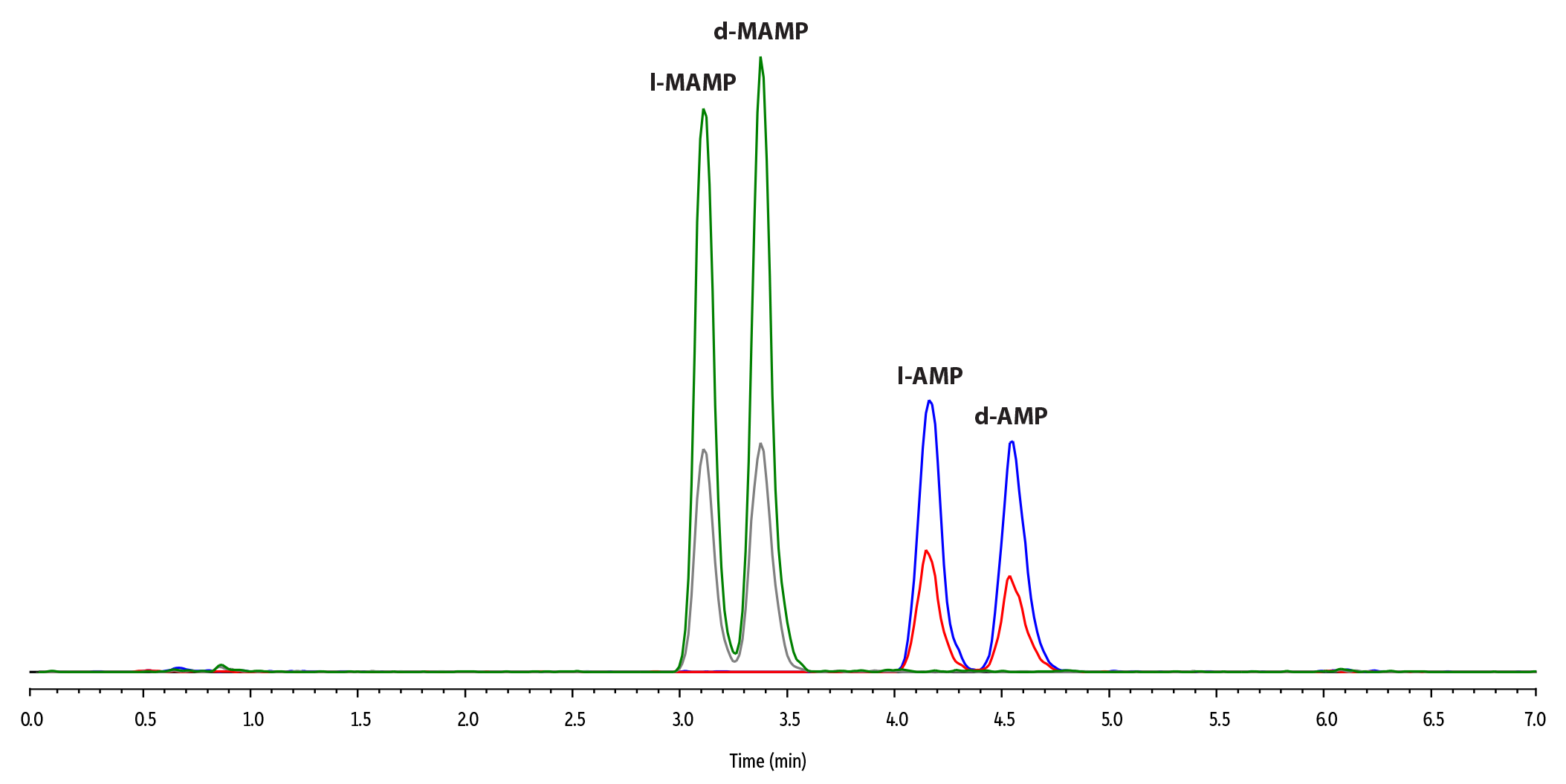
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||||||||||
| Temp.: | 35 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Conc.: | 500 ng/mL in urine | ||||||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||||||||||
| B: | 0.1% Formic acid in methanol | ||||||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | HPLC |
| Sample Preparation | A 500 ng/mL standard (d- and l-amphetamines and methamphetamines) was prepared in pooled urine. 50 µL of the standard was aliquoted into a microcentrifuge tube. 10 µL of a working internal standard (20 µg/mL (±)-amphetamine-D11 and (±)-methamphetamine-D11 in water) and 20 µL of 1M NaHCO3 was added and vortexed at 3000 rpm for 10 seconds. After vortexing, 100 µL of 0.1% (w/v) Marfey's reagent (1-fluoro-2-4-dinitrophenyl-5-L-alanine amide) in acetone was added, vortexed, and heated at 45 °C for 1 hour. Samples were allowed to cool to room temperature before the addition of 40 µL of 1M HCl in water. The sample was then vortexed and evaporated to dryness under nitrogen at 45 °C. Samples were reconstituted in 1 mL of 40:60 water:methanol (v/v) and filtered using Thomson SINGLE StEP standard filter vials cat.# 25893 prior to analysis. |
| Notes | Thomson SINGLE StEP standard filter vials cat.# 25893 were used to produce this chromatogram, but have since been discontinued. For assistance choosing a replacement for this application, contact Restek Technical Service or your local Restek representative. |
Selectivity and Specificity
Consumption of over-the-counter (OTC) drugs that contain l-methamphetamine can result in a high-intensity l-enantiomer peak in urine, which may make it difficult to identify very low concentrations of the illegal enantiomer (d-methamphetamine), leading to false negative results. The method developed here was found to be highly specific and selective with good chiral resolution even when evaluated at extreme concentrations, such as high l- enantiomer (5000 ng/mL) with low d- enantiomer (50 ng/mL) for both amphetamines and methamphetamines in urine (Figure 4).
Figure 4: Highly selective chromatographic results were obtained even at extreme concentrations.
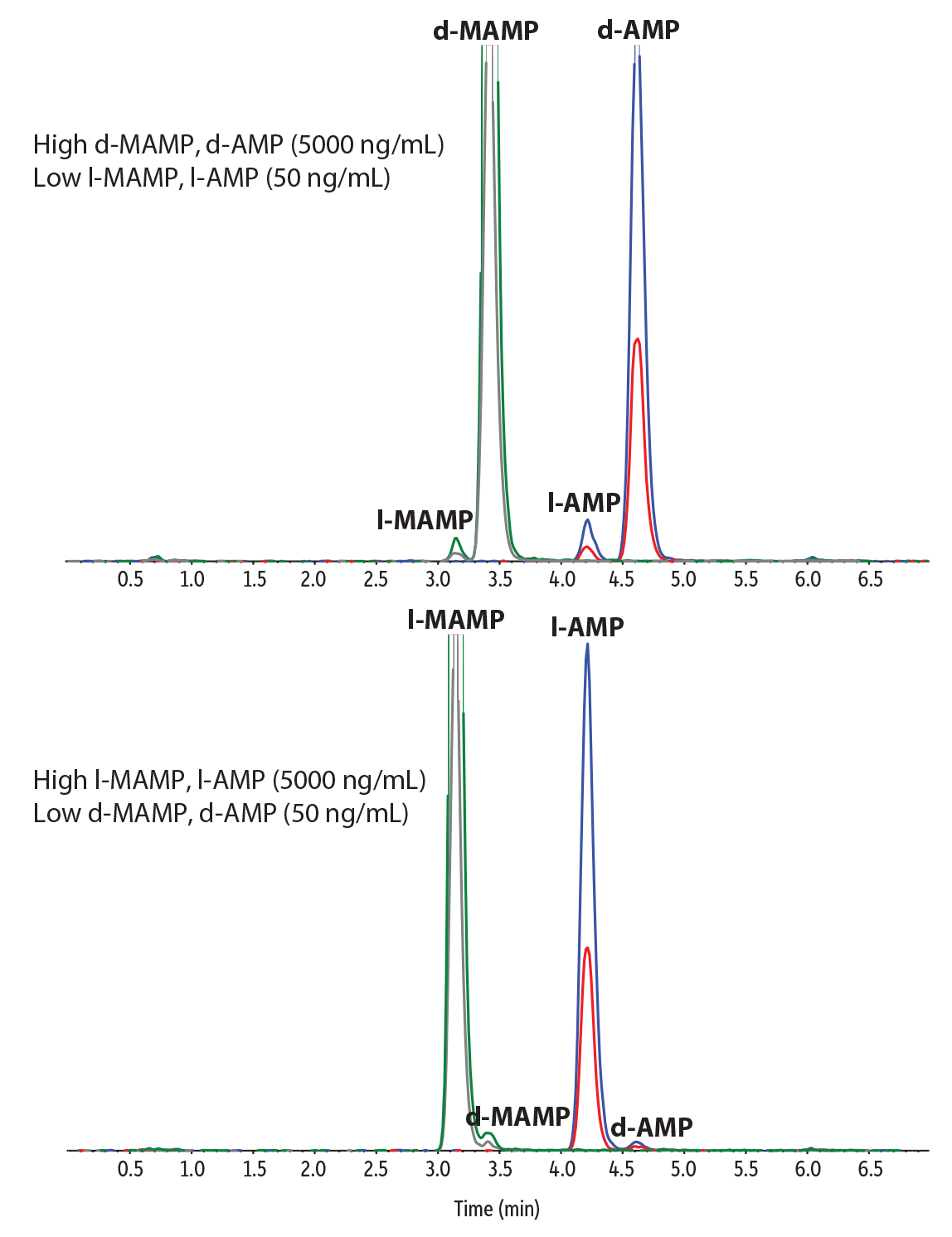
| Peaks | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|
| 1. | l-Methamphetamine (l-MAMP) | 400.3 | 339.0 | 323.8 |
| 2. | d-Methamphetamine (d-MAMP) | 400.3 | 339.0 | 323.8 |
| 3. | l-Amphetamine (l-AMP) | 386.1 | 325.0 | 308.0 |
| 4. | d-Amphetamine (d-AMP) | 386.1 | 325.0 | 308.0 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||||||||||
| Temp.: | 35 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | 40:60 Water:methanol (v/v) | ||||||||||||||||||||||||||||
| Conc.: | 50-5000 ng/mL in urine | ||||||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||||||||||
| B: | 0.1% Formic acid in methanol | ||||||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Instrument | HPLC |
| Sample Preparation | Two multi-analyte standards were prepared in pooled human urine: one at 5000 ng/mL d-MAMP-d-AMP + 50 ng/mL l-MAMP-l-AMP, and the other at 5000 ng/mL l-MAMP-l-AMP + 50 ng/mL d-MAMP-d-AMP. 50 µL from each of the standards was aliquoted into two separate microcentrifuge tubes. 10 µL of a working internal standard (20 µg/mL (±)-amphetamine-D11 and (±)-methamphetamine-D11 in water) and 20 µL of 1M NaHCO3 were added and vortexed at 3000 rpm for 10 seconds, respectively. After vortexing, 100 µL of 0.1% (w/v) Marfey's Reagent (1-fluoro-2-4-dinitrophenyl-5-L-alanine amide) in acetone was added to both the tubes, vortexed, and heated at 45 °C for 1 hour. Samples were allowed to cool to room temperature before the addition of 40 µL of 1M HCl in water. The samples were then vortexed and evaporated to dryness under nitrogen at 45 °C. Samples were reconstituted in 1 mL of 40:60 water:methanol (v/v) and filtered using Thomson SINGLE StEP standard filter vials cat.# 25893 prior to analysis. |
| Notes | Thomson SINGLE StEP standard filter vials cat.# 25893 were used to produce this chromatogram, but have since been discontinued. For assistance choosing a replacement for this application, contact Restek Technical Service or your local Restek representative. |
Linearity
Using 1/x weighted linear regression, all four analytes showed acceptable linearity with r2 values of 0.998 or greater (Figure 5). In addition, the %deviation from nominal concentration was <15%, in all three accuracy and precision experiments (Table II).
Figure 5: Standard Curves.
l-MAMP (1/x), y= 0.000454x+0.00747 (r2 = 0.9991)
d-MAMP (1/x), y= 0.00049x+0.00822 (r2 = 0.9985)
l-AMP (1/x), y= 0.000423x+0.00365 (r2 = 0.9982)
d-AMP (1/x), y= 0.000423x+0.0021 (r2 = 0.9991)
Accuracy and Precision
Precision and accuracy analyses were performed on three different days. Method accuracy was demonstrated by recovery values within 10% of the nominal concentrations for low, mid, and high QC levels and within 15% for the LLOQ. The %RSD was 1–8% and 0.6–8% for intraday and interday results, respectively, indicating acceptable method precision (Table II). Because deuterated internal standards were used for each enantiomer, the standards and target analytes experienced similar enhancements, which ensured accurate and reliable quantitative results were obtained.
Table II: Accurate and precise results were obtained for the analysis of amphetamines by LC-MS/MS in urine (interday comparison).
| Analyte |
QC LLOQ (50 ng/mL) |
QC Low (125 ng/mL) |
QC Mid (700 ng/mL) |
QC High (4000 ng/mL) |
||||
|---|---|---|---|---|---|---|---|---|
|
Avg. Accuracy (%) |
Precision (%RSD) |
Avg. Accuracy (%) |
Precision (%RSD) |
Avg. Accuracy (%) |
Precision (%RSD) |
Avg. Accuracy (%) |
Precision (%RSD) |
|
|
l-MAMP |
86.6 |
3.40 |
99.2 |
1.49 |
104 |
3.11 |
106 |
1.64 |
|
d-MAMP |
87.2 |
3.39 |
102 |
6.08 |
107 |
1.72 |
106 |
1.40 |
|
l-AMP |
99.8 |
7.97 |
104 |
6.03 |
109 |
2.02 |
106 |
0.640 |
|
d-AMP |
98.8 |
5.69 |
99.7 |
6.80 |
103 |
3.38 |
101 |
0.830 |
Conclusion
A highly selective method for the analysis of amphetamines by LC-MS/MS in urine was successfully developed. Separation was achieved within a total analysis time of 7 minutes and quantitation in urine was performed across a linear range of 50-5000 ng/mL. Verification experiments across this range demonstrated reliable analysis of d- and l-amphetamine and methamphetamine enantiomers in a workflow and timeframe suitable for high-throughput clinical and forensic toxicology labs. In addition, the method allows licit vs. illicit methamphetamine to be distinguished and quantified without the expense of chiral columns or dedicated instruments.
References
- M.N. Newmeyer, M. Concheiro, M.A. Huestis, Rapid quantitative chiral amphetamines liquid chromatography-tandem mass spectrometry method in plasma and oral fluid with a cost-effective chiral derivatizing reagent, J Chromatogr A 1358 (5) (2014) 68–74. https://doi.org/10.1016/j.chroma.2014.06.096
- B.S. Foster, D.D. Gilbert, A. Hutchaleelaha, M. Mayersohn, Enantiomeric determination of amphetamine and methamphetamine in urine by precolumn derivatization with Marfey's reagent and HPLC, J Analytical Toxicology 22 (4) (1998) 265-269. https://doi.org/10.1093/jat/22.4.265

