Analysis of Furan and Alkylfurans in Food Commodities by SPME Arrow GC-MS
Abstract
In this study, SPME Arrow sample preparation was tested for the analysis of furan and alkylfurans in baby formula and coffee samples. Optimized conditions were established for GC-MS analysis using a PAL CTC autosampler and an Rxi-624Sil MS column. Results demonstrated the method may be suitable for diverse sample types containing both high and low concentrations of furan and alkylfurans. This approach could allow food safety labs to improve productivity by using SPME Arrow as a single sample preparation approach compared to methods that use headspace alone or SPME for low-concentration samples and headspace for high-concentration samples.
Introduction
Analysis of furan and alkylfurans is emerging as a growth area in food safety testing due to increasing recognition of their potential health risks. These compounds form through a diverse array of chemical reactions that occur during heating processes, such as roasting or sterilization, so exposure can occur through a wide range of foods. Exposure has not been well characterized, so in early 2022, the European Commission adopted a recommendation calling for member states to monitor furan, 2-methylfuran, and 3-methylfuran in particular foods [1]. The agency also advised monitoring non-methylfurans, such as 2,5-dimethylfuran, 2-pentylfuran, and 2-ethylfuran, if methods are available that have been demonstrated to be reliable for this purpose.
Furan and alkylfurans are volatile compounds that can be analyzed by static headspace (HS) and/or solid phase microextraction (SPME) in combination with GC-MS. Static headspace GC-MS is typically used with samples that contain high levels of furan and alkylfurans, such as coffee, but it may not offer adequate sensitivity for low concentration samples. SPME is more sensitive and better for low-level samples, but traditional SPME fibers are very fragile and not well suited to high-throughput environments. To meet regulatory expectations efficiently, food safety labs need a simple, fast, cost-effective workflow that performs well for a wide range of analyte concentrations in different sample types.
For this reason, we decided to evaluate the performance of SPME Arrows to determine if a single approach could be used for the analysis of furan and alkylfurans. SPME Arrows are second-generation SPME devices that are mechanically enhanced with a sheath and inner rod to prevent breakage; they also have higher phase volumes to provide more loading capacity (Figure 1). In this study, based on an initial comparison of sample preparation techniques, we developed an optimized SPME Arrow GC-MS method for the analysis of furan and alkylfurans across a range of concentrations in both coffee and baby formula.
| SPME Arrows | SPME Fibers |
Experimental
Standard Solutions
For each target analyte, 1 mg/mL solutions were prepared in methanol by spiking pure standards using a glass syringe. Then, 100 ppm stock solutions, one containing all the target analytes and one containing all the internal standards, were prepared in methanol. For the calibration curve covering a low concentration range, 0.1 and 0.5 µg/mL working solutions were prepared by spiking 10 and 50 µL of the 100 ppm stock, respectively, in 10 mL of LC-MS grade water. An internal standard solution of 1 µg/mL was prepared by adding 100 µL of the 100 ppm stock solution to 9.9 mL of water. For the analysis of coffee samples, a high-concentration calibration curve was used. For this purpose, aqueous working solutions at 10 and 25 µg/mL, for target analytes and internal standards, respectively, were made by spiking the original individual methanolic solutions directly into water. A 0.5 µg/mL solution was prepared by diluting 500 µL of the 10 µg/mL solution in water until reaching a volume of 10 mL.
Sample Preparation
Baby formula and instant coffee samples were purchased at a local grocery store. Preliminary experiments comparing headspace, traditional SPME fibers, and SPME Arrows were run to establish a sample preparation procedure that would be suitable for a wide range of analyte concentrations. Parameters tested included coating chemistry, incubation time and temperature, and extraction time [2,3,4].
Based on the results of the initial sample preparation experiments, SPME Arrow was selected as the best approach and was used under the optimized conditions shown in Table I for subsequent method evaluation experiments. All samples and calibration solutions were prepared in 20 mL glass vials and extracted and analyzed by automated headspace-SPME using a 120 µm wide carbon range/PDMS Restek PAL SPME Arrow (cat.# 27487) assembled in a PAL CTC autosampler.
Table I: Sample Preparation Conditions for Baby Formula and Coffee Samples
|
Sample |
Baby Formula |
Coffee |
|
Amount |
0.5 g |
|
|
Volume of 30% NaCl solution added |
10 mL |
5 mL |
|
Volume of internal standard solution added |
50 µL (1 µg/mL solution) |
40 µL (25 µg/mL solution) |
|
Incubation time |
10 min |
|
|
Incubation and extraction temperature |
50 °C |
|
|
Agitation |
250 rpm |
|
|
HS-SPME extraction time |
10 min |
1 min |
|
Desorption temperature |
280 °C |
|
|
Desorption time |
1 min |
|
Calibration Curves
Two calibration curves were prepared in 30% sodium chloride solution: one for low concentration analysis of furan and alkylfurans in baby formula, and one for the quantitation of high concentrations of these target analytes in coffee. For the low calibration range, vials were filled with 10 mL of sodium chloride solution, whereas for the high calibration range, 5 mL of solution were added. Analytes were spiked by adding different volumes of working solutions to the calibration vials, as shown in Table II. Internal standard solutions were added to each calibration vial (50 µL of the 1 µg/mL solution for the low concentration calibration curve, and 40 µL of the 25 µg/mL solution for the high concentration calibration curve). To account for variation in recoveries due to matrix differences, it was essential to use isotopically labeled analogues for almost every analyte (except for 2- and 3-methylfuran because 2-methylfuran-d6 worked for both compounds). Calibration curves were constructed by plotting analyte area/internal standard average area ratios (n=2) versus spiked concentration.
Table II: Calibration Solutions
|
|
Low Concentration Calibration Curve |
High Concentration Calibration Curve |
||
|
ng of analyte in vial |
µL of working solution added (solution concentration) |
ng of analyte in vial |
µL of working solution added (solution concentration) |
|
|
Level 1 |
1.25 |
12.5 (0.1 µg/mL) |
25 |
50 (0.5 µg/mL) |
|
Level 2 |
2.5 |
25 (0.1 µg/mL) |
50 |
100 (0.5 µg/mL) |
|
Level 3 |
5 |
10 (0.5 µg/mL) |
100 |
10 (10 µg/mL) |
|
Level 4 |
10 |
20 (0.5 µg/mL) |
200 |
20 (10 µg/mL) |
|
Level 5 |
20 |
40 (0.5 µg/mL) |
500 |
50 (10 µg/mL) |
|
Level 6 |
50 |
100 (0.5 µg/mL) |
1000 |
100 (10 µg/mL) |
|
Level 7 |
100 |
200 (0.5 µg/mL) |
2000 |
200 (10 µg/mL) |
|
Level 8 |
150 |
300 (0.5 µg/mL) |
4000 |
400 (10 µg/mL) |
|
Level 9 |
|
|
8000 |
800 (10 µg/mL) |
Method Verification Samples
To evaluate method performance, baby formula and coffee samples were spiked at two concentration levels based on the standard method performance requirements listed by the AOAC [6]. Baby formula samples were spiked at 5 and 50 µg/kg (n=3), and coffee samples were spiked at 1000 and 4000 µg/kg (n=3). Sample blanks spiked only with internal standards were also analyzed (n=3).
Chromatographic Method
GC-MS analysis of furan and alkylfurans was performed on an Agilent 7890B GC paired with a 5977B MSD. GC-MS method details are shown in Tables III and IV. To enable the analysis of highly concentrated coffee samples with the wide carbon range SPME Arrows, three method parameters were modified: the GC split was set to 100:1 (with a split flow rate of 140 mL/min); the extraction time was set to 1 min; and the volume of sodium chloride solution was adjusted to 5 mL. Note that a 1.8 mm ID inlet liner was used because the wider bore diameter is necessary to accommodate the width of SPME Arrows.
Table III: GC-MS Instrument Conditions
|
Column |
Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|
Injection Mode |
Split (10:1 for baby formula and 100:1 for coffee) |
|
Liner |
Topaz 1.8 mm ID SPME/straight liner (cat.# 23280) |
|
Inj. Temp. |
280 °C |
|
Split Flow |
· Baby formula: 14.0 mL/min (10:1) · Coffee: 140 mL/min (100:1) |
|
Purge Flow |
5 mL/min |
|
Oven |
35 °C (hold 3 min) to 75 °C by 8 °C/min, then to 200 °C by 25°C/min (hold 1 min) |
|
Carrier Gas |
He, constant flow |
|
Flow Rate |
1.40 mL/min |
|
Analyzer |
MS (quadrupole) |
|
Acquisition Type |
SIM |
|
Ionization Mode |
EI (70 eV) |
|
Transfer Line Temp. |
280 °C |
|
Source Temp. |
325 °C |
|
Quadrupole Temp. |
200 °C |
|
Solvent delay |
2.2 min |
Table IV: MS Parameters (SIM mode)
|
Segment Starting Time (min) |
Compound (tR, min) |
Ions |
Dwell Time (ms) |
|
2.2 |
Furan (2.447) |
39 |
50 |
|
68* |
|||
|
Furan-d4 (2.428) |
42 |
||
|
72* |
|||
|
4.2 |
2-Methylfuran (4.536) |
53 |
30 |
|
81 |
|||
|
82* |
|||
|
3-Methylfuran (4.846) |
53 |
30 |
|
|
81 |
|||
|
82* |
|||
|
2-Methylfuran-d6 (4.464) |
58 |
30 |
|
|
88* |
|||
|
6.6 |
2-Ethylfuran (7.100) |
53 |
30 |
|
81* |
|||
|
96 |
|||
|
2-Ethylfuran-d5 (7.001) |
55 |
30 |
|
|
101* |
|||
|
2,5-Dimethylfuran (7.243) |
67 |
30 |
|
|
95* |
|||
|
2,5-Dimethylfuran-d3 (7.179) |
84 |
30 |
|
|
99* |
|||
|
10.6 |
2-Pentylfuran (11.570) |
81 |
30 |
|
138* |
30 |
||
|
2-Pentylfuran-d11 (11.501) |
83 |
30 |
|
|
149* |
30 |
*Quantifier ions
Results and Discussion
Comparison of Headspace, SPME Fiber, and SPME Arrow Sample Preparation
A comparison of various sampling strategies was conducted at the beginning of the study. SPME Arrow (wide carbon range coating); traditional SPME fibers (wide carbon range coating); and headspace sampling were evaluated in terms of area counts after sampling from 30% sodium chloride solution (10 mL) spiked with all analytes (20 ng of each analyte). As can be seen in Figure 2, the SPME Arrow provided significantly higher responses for all target analytes compared to headspace or traditional SPME fibers. Compared to SPME fibers, SPME Arrows have the additional advantage of a stainless-steel sheath and stabilizing inner rod, which prevent breakage during use. Based on the response data and rugged, steel construction, SPME Arrow was chosen for the development of a single sample preparation GC-MS method.
Chromatographic Performance
An Rxi-624Sil MS column (30 m, 0.25 mm ID, 1.40 µm [cat.# 13868]) was used for the analysis of furan and alkylfurans because it is highly selective for volatile organics and works well for both SPME and headspace methods. The column and optimized conditions provided good chromatographic separation of all compounds, and example chromatograms for a solvent standard and spiked samples are provided in Figures 3-5. Of particular note, chromatographic separation between 2,5-dimethylfuran and 2-ethylfuran (isomeric compounds) and between 2,5-dimethylfuran-d3 and 2-ethylfuran was achieved, which allows accurate identification and quantitation (Figure 4). Chromatographic separation is important because 2,5-dimethylfuran-d3 and 2-ethylfuran share most of the ions of their mass spectra (except for ions m/z 95 and 96) and, if they are not separated, 2,5-dimethylfuran-d3 can contribute to the most abundant qualifier ions of 2-ethylfuran (m/z 81 and 53). All compounds were adequately separated in a 14-minute analysis.
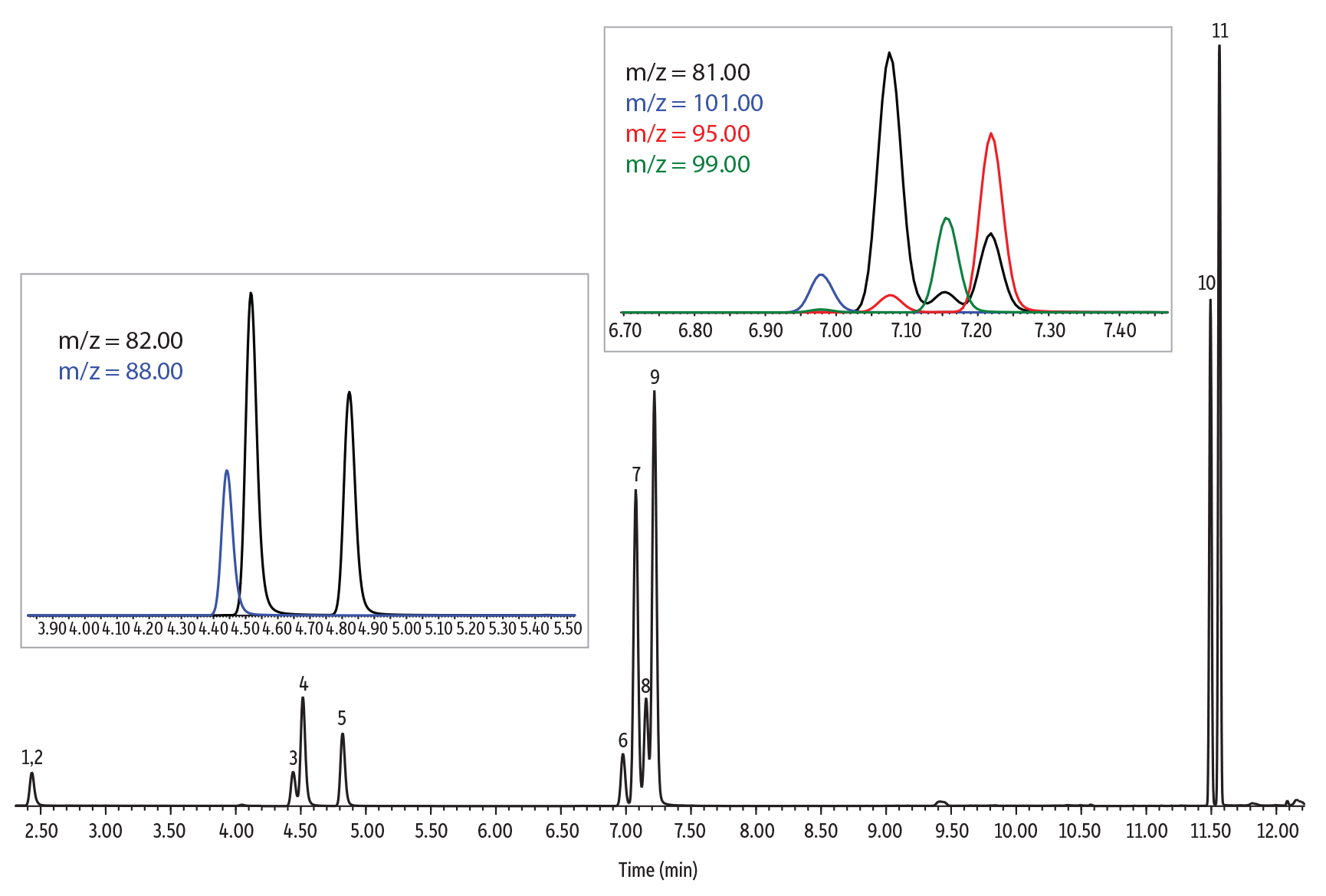
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 1. | Furan-d4 | 2.428 | 72 | 42 | 50 |
| 2. | Furan | 2.447 | 68 | 39 | 50 |
| 3. | 2-Methylfuran-d6 | 4.464 | 88 | 58 | 30 |
| 4. | 2-Methylfuran | 4.536 | 82 | 53 | 30 |
| 5. | 3-Methylfuran | 4.846 | 82 | 53 | 30 |
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 6. | 2-Ethylfuran-d5 | 7.001 | 101 | 55 | 30 |
| 7. | 2-Ethylfuran | 7.100 | 81 | 96 | 30 |
| 8. | 2,5-Dimethylfuran-d3 | 7.179 | 99 | 84 | 30 |
| 9. | 2,5-Dimethylfuran | 7.243 | 95 | 67 | 30 |
| 10. | 2-Pentylfuran-d11 | 11.501 | 149 | 83 | 30 |
| 11. | 2-Pentylfuran | 11.570 | 138 | 81 | 30 |
| Column | Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|---|---|
| Standard/Sample | |
| Diluent: | N/A |
| Conc.: | 10 ng/mL |
| Injection | split (split ratio 10:1) |
| Liner: | Topaz 1.8 mm ID straight/SPME inlet liner (cat.# 23280) |
| Inj. Temp.: | 280 °C |
| Split Vent Flow Rate: | 14 mL/min |
| Oven | |
| Oven Temp.: | 35 °C (hold 3 min) to 75 °C at 8 °C/min to 200 °C at 25 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Detector | MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Analyzer Type: | Quadrupole |
| Source Temp.: | 325 °C |
| Quad Temp.: | 200 °C |
| Electron Energy: | 70 eV |
| Tune Type: | PFTBA |
| Ionization Mode: | EI |
| Instrument | Agilent 7890B GC & 5977B MSD |
| Sample Preparation | Data was collected by extracting via HS-SPME from a 20 mL vial (cat.# 23083) capped with a magnetic screw-thread cap (cat.# 23091). The vial contained 10 mL of sodium chloride solution spiked with 100 ng of each analyte and internal standard. A Restek PAL SPME Arrow (120 µm carbon wide range [WR]/PDMS; cat.# 27487) was used. SPME Arrow sampling conditions: 10 min extraction time, 10 min incubation at 50 °C, 1 min desorption at 280 °C, agitation at 250 rpm. |
| Notes | A furan/alkylfurans standard (cat.# 33334) is now available. |
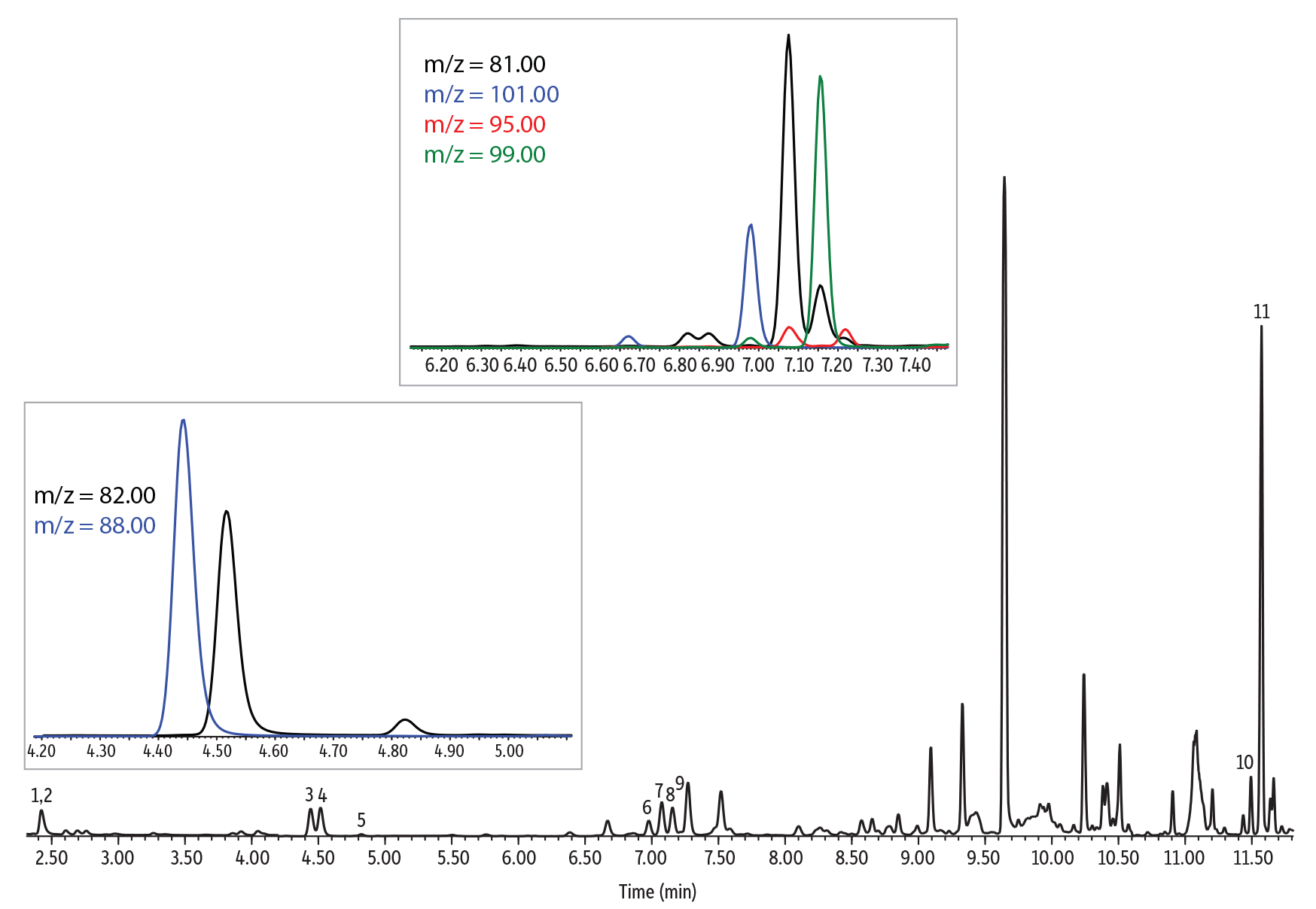
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 1. | Furan-d4 | 2.428 | 72 | 42 | 50 |
| 2. | Furan | 2.447 | 68 | 39 | 50 |
| 3. | 2-Methylfuran-d6 | 4.464 | 88 | 58 | 30 |
| 4. | 2-Methylfuran | 4.536 | 82 | 53 | 30 |
| 5. | 3-Methylfuran | 4.846 | 82 | 53 | 30 |
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 6. | 2-Ethylfuran-d5 | 7.001 | 101 | 55 | 30 |
| 7. | 2-Ethylfuran | 7.100 | 81 | 96 | 30 |
| 8. | 2,5-Dimethylfuran-d3 | 7.179 | 99 | 84 | 30 |
| 9. | 2,5-Dimethylfuran | 7.243 | 95 | 67 | 30 |
| 10. | 2-Pentylfuran-d11 | 11.501 | 149 | 83 | 30 |
| 11. | 2-Pentylfuran | 11.570 | 138 | 81 | 30 |
| Column | Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|---|---|
| Standard/Sample | |
| Diluent: | N/A |
| Conc.: | 5 µg/kg |
| Injection | split (split ratio 10:1) |
| Liner: | Topaz 1.8 mm ID straight/SPME inlet liner (cat.# 23280) |
| Inj. Temp.: | 280 °C |
| Split Vent Flow Rate: | 14 mL/min |
| Oven | |
| Oven Temp.: | 35 °C (hold 3 min) to 75 °C at 8 °C/min to 200 °C at 25 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Detector | MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Analyzer Type: | Quadrupole |
| Source Temp.: | 325 °C |
| Quad Temp.: | 200 °C |
| Electron Energy: | 70 eV |
| Tune Type: | PFTBA |
| Ionization Mode: | EI |
| Instrument | Agilent 7890B GC & 5977B MSD |
| Sample Preparation | Data was collected by extracting via HS-SPME from a 20 mL vial (cat.# 23083) capped with a magnetic screw-thread cap (cat.# 23091). The vial contained 0.5 g of baby formula and 10 mL of sodium chloride solution spiked with 2.5 ng of each analyte (5 ng/g final concentration). Internal standards were spiked at 100 ng/g. A Restek PAL SPME Arrow (120 µm carbon wide range [WR]/PDMS; cat.# 27487) was used. SPME Arrow sampling conditions: 10 min extraction time, 10 min incubation at 50 °C, 1 min desorption at 280 °C, agitation at 250 rpm. |
| Notes | A furan/alkylfurans standard (cat.# 33334) is now available. |
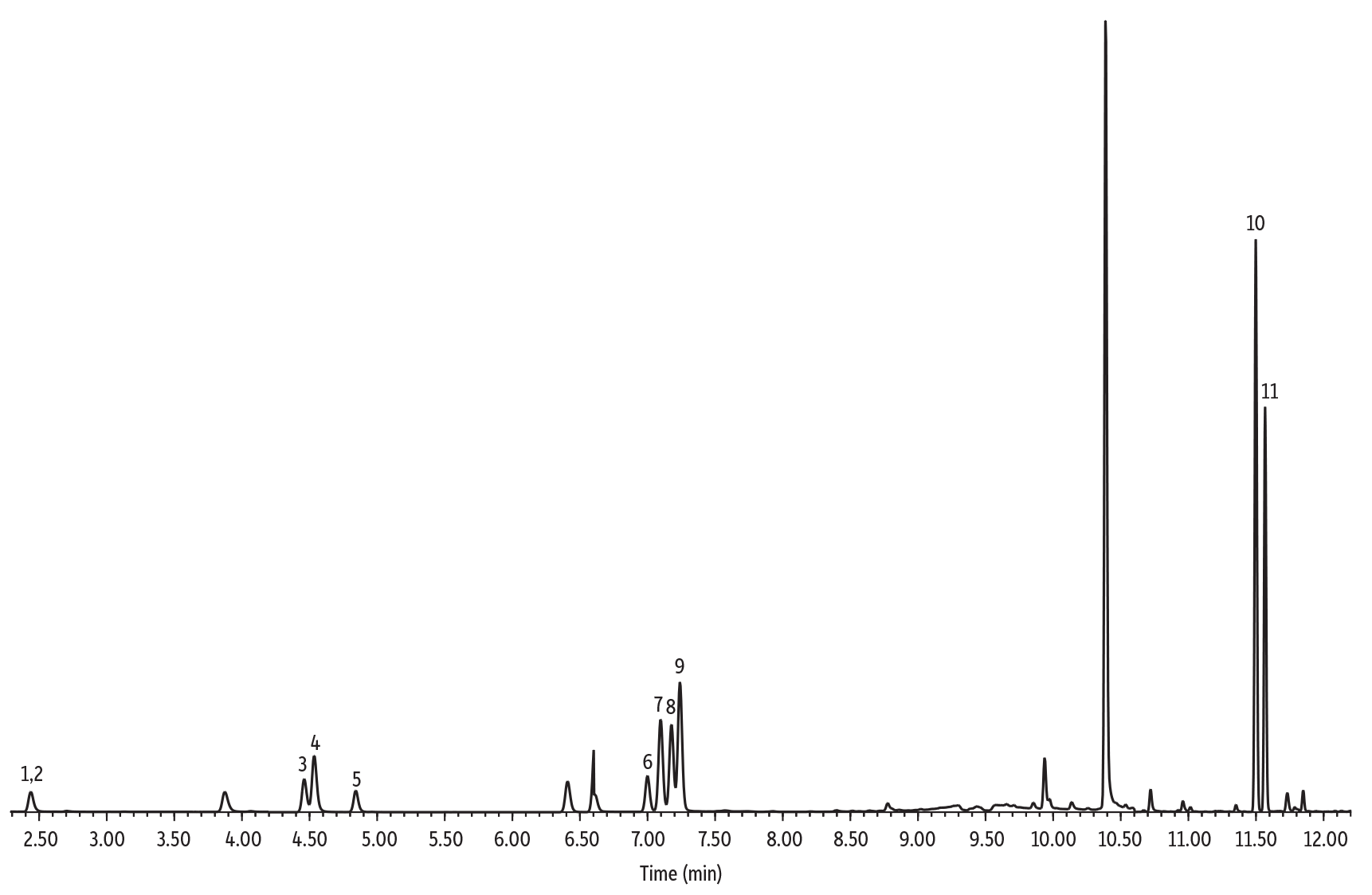
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 1. | Furan-d4 | 2.428 | 72 | 42 | 50 |
| 2. | Furan | 2.447 | 68 | 39 | 50 |
| 3. | 2-Methylfuran-d6 | 4.464 | 88 | 58 | 30 |
| 4. | 2-Methylfuran | 4.536 | 82 | 53 | 30 |
| 5. | 3-Methylfuran | 4.846 | 82 | 53 | 30 |
| Peaks | tR (min) | Ion (Quantifier) | Ion (Qualifier) | Dwell Time (ms) | |
|---|---|---|---|---|---|
| 6. | 2-Ethylfuran-d5 | 7.001 | 101 | 55 | 30 |
| 7. | 2-Ethylfuran | 7.100 | 81 | 96 | 30 |
| 8. | 2,5-Dimethylfuran-d3 | 7.179 | 99 | 84 | 30 |
| 9. | 2,5-Dimethylfuran | 7.243 | 95 | 67 | 30 |
| 10. | 2-Pentylfuran-d11 | 11.501 | 149 | 83 | 30 |
| 11. | 2-Pentylfuran | 11.570 | 138 | 81 | 30 |
| Column | Rxi-624Sil MS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 13868) |
|---|---|
| Standard/Sample | |
| Diluent: | N/A |
| Conc.: | 1000 µg/kg |
| Injection | split (split ratio 100:1) |
| Liner: | Topaz 1.8 mm ID straight/SPME inlet liner (cat.# 23280) |
| Inj. Temp.: | 280 °C |
| Split Vent Flow Rate: | 140 mL/min |
| Oven | |
| Oven Temp.: | 35 °C (hold 3 min) to 75 °C at 8 °C/min to 200 °C at 25 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Detector | MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Analyzer Type: | Quadrupole |
| Source Temp.: | 325 °C |
| Quad Temp.: | 200 °C |
| Electron Energy: | 70 eV |
| Tune Type: | PFTBA |
| Ionization Mode: | EI |
| Instrument | Agilent 7890B GC & 5977B MSD |
| Sample Preparation | Data was collected by extracting via HS-SPME from a 20 mL vial (cat.# 23083) capped with a magnetic screw-thread cap (cat.# 23091). The vial contained 0.5 g of instant coffee and 5 mL of sodium chloride solution spiked with 500 ng of each analyte (1000 ng/g final concentration). Internal standards were spiked at 2000 ng/g. A Restek PAL SPME Arrow (120 µm carbon wide range [WR]/PDMS; cat.# 27487) was used. SPME Arrow sampling conditions: 1 min extraction time, 10 min incubation at 50 °C, 1 min desorption at 280 °C, agitation at 250 rpm. |
| Notes | A furan/alkylfurans standard (cat.# 33334) is now available. |
Linearity
Calibration curves were constructed for all the target analytes in sodium chloride solution (30%) using appropriate deuterated analogues for each compound. The use of isotopically labeled internal standards was essential to account for differences in analyte recovery from sample matrices versus from spiked sodium chloride solution. The quantitation strategy followed in this work was a slightly modified version of the method described by Frank et al. in 2020 [5]. The low concentration calibration curve ranged from 1.25 to 150 ng of analytes in vial (8 calibration points) while the high concentration calibration curve ranged from 25 to 8000 ng of analyte in vial (9 calibration points). As can be seen in Figure 6, all analytes showed good linearity within the tested ranges, and all the coefficients of determination were above 0.99. For pentylfuran, the low concentration calibration curve ranged from 1.25 to 100 ng, and the high concentration calibration curve was linear from 25 to 2000 ng in vial.
Method Verification in Baby Formula and Coffee Samples
Baby formula and coffee both had significant concentrations of some of the analytes of interest in the blank samples. Baby formula was analyzed using the low concentration calibration curve, whereas instant coffee was analyzed using the high concentration calibration curve. Table V and VI present a summary of the collected results. All results for baby formula passed the 80-110% recovery criteria, except for 3-methylfuran, which had a recovery of 113% in the low spike. For coffee, all low concentration spikes passed, but some high bias was observed for the high concentration spikes. While further investigation into sample size, sample handling, and dilution could be done in future experiments, recoveries were still fairly close to the 110% upper limit. This indicates that with some modification SPME Arrow is likely to be an effective approach for high concentration samples as well as low concentration samples.
Table V: Analysis of furan and alkylfurans in baby formula.
|
Analyte |
Blank (n=3) |
Low concentration (n=3), 5 µg/kg* |
High concentration (n=3), 50 µg/kg* |
|||
|
Concentration, µg/kg |
RSD, % |
Accuracy, % |
RSD, % |
Accuracy, % |
RSD, % |
|
|
Furan |
16 |
1 |
110 |
2 |
94 |
4 |
|
2-Methylfuran |
60 |
2 |
97 |
2 |
100 |
3 |
|
3-Methylfuran |
- |
- |
113 |
3 |
107 |
6 |
|
2-Ethylfuran |
67 |
2 |
93 |
3 |
105 |
5 |
|
2,5-Dimethylfuran |
- |
- |
101 |
4 |
97 |
14 |
|
2-Pentylfuran |
219 |
3 |
87 |
11 |
108 |
12 |
*Accuracy was determined as follows: ((measured concentration – concentration in blank)/spiked concentration)*100
Table VI: Analysis of furan and alkylfurans in instant coffee.
|
Analyte |
Blank (n=3) |
Low concentration (n=3), 1000 µg/kg* |
High concentration (n=3), 4000 µg/kg* |
|||
|
Concentration, µg/kg |
RSD, % |
Accuracy, % |
RSD, % |
Accuracy, % |
RSD, % |
|
|
Furan |
394 |
10 |
87 |
2 |
125 |
3 |
|
2-Methylfuran |
843 |
10 |
94 |
3 |
116 |
2 |
|
3-Methylfuran |
96 |
10 |
87 |
2 |
119 |
3 |
|
2-Ethylfuran |
29 |
11 |
93 |
2 |
115 |
5 |
|
2,5-Dimethylfuran |
46 |
11 |
98 |
2 |
120 |
4 |
|
2-Pentylfuran |
- |
- |
83 |
3 |
83 |
11 |
*Accuracy was determined as follows: ((measured concentration – concentration in blank)/spiked concentration)*100
Conclusion
Analysis of furan and alkylfurans is complicated by a diverse array of sample types that can contain the target compounds at a wide range of concentrations. Headspace and traditional SPME fibers can be used for high and low concentration samples respectively, but this study sought to establish a single approach that could be adapted for all analyte levels. The method developed here, based on SPME Arrow sample preparation, was evaluated in matrices spiked at both low and high concentration levels: 5 and 50 µg/kg for baby formula, and 1000 and 4000 µg/kg for coffee. Satisfactory results in terms of linearity, accuracy, and precision were obtained in most cases. It is noted that for the analysis of highly concentrated samples; different split (1:100); extraction time (1 min); and sodium chloride solution (30%) (5 mL) conditions should be used, and further optimization may be necessary. Use of this SPME Arrow GC-MS method may allow labs to improve productivity by using a single sample preparation technique that can be adjusted to accommodate both high and low concentration samples.
References
- European Commission recommendation 2022/495, On monitoring the presence of furan and alkylfurans in food, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32022H0495&from=EN#ntr1-L_2022100EN.01006001-E0001
- Reyes-Garcés, Analysis of furan and alkylfurans in food (part 1): choosing GC-MS conditions and a sample preparation technique, ChromaBLOGraphy, Restek Corporation, 2022.
- Reyes-Garcés, Analysis of furan and alkylfurans in food (part 2): optimizing experimental conditions for the analysis of food matrices using the SPME Arrow, ChromaBLOGraphy, Restek Corporation, 2022.
- Reyes-Garcés, Analysis of furan and alkylfurans in food samples (part 3): Adjusting the experimental conditions for the analysis of samples with high concentrations of analytes and preparing calibration curves, ChromaBLOGraphy, Restek Corporation, 2022.
- Frank, M. Dubois, J.F. Huertas Pérez, Detection of furan and five alkylfurans, including 2-pentylfuran, in various food matrices, J. Chromatogr. A, 1622 (2020) 461119. https://doi.org/10.1016/j.chroma.2020.461119
- AOAC SMPR 2019.004, Standard method performance requirements (SMPRs) for furan and alkyl furans in coffee, baby foods, infant formula, cereals, and fruit juices, final version: October 3, 2019, AOAC International, Rockville, MD, U.S. https://www.aoac.org/wp-content/uploads/2019/09/SMPR_Furans_v6-For-Comment.pdf

