Analytical Method for Polycyclic Aromatic Hydrocarbons (PAHs) in Yerba Mate Tea Using Modified QuEChERS, Solid Phase Extraction and GC-TOFMS and GC-MS/MS
Abstract
Polycyclic aromatic hydrocarbons (PAHs) are toxic compounds found in some foods, especially those that are smoked, roasted, grilled, or dried during preparation. Yerba mate (Ilex paraguariensis) tea is of particular interest because of relatively high PAH levels and proposed links between yerba mate tea and health problems. While classic sample extraction methods yield excellent results for PAHs in tea, these techniques are time consuming and costly. A much less resource-intensive modified QuEChERS extraction and silica solid phase extraction (SPE) sample cleanup method was developed and yielded good quantitative recoveries for PAHs in yerba mate tea. Chromatographic separation of EFSA PAH4 compounds and isobaric interferences was optimized on a high-phenyl stationary phase using both GC-TOFMS with hydrogen carrier gas and GC-MS/MS. Incurred values of PAHs determined via GC-TOFMS and GC-MS/MS compared favorably. Total levels of EFSA PAH4 compounds were relatively high with respect to other foods and ranged from approximately 200 to 800 ng/g in dry tea.
Introduction
Traditionally, mate tea is brewed from loose yerba mate (Ilex paraguariensis) leaves and stems in hot water and drunk from a gourd through a metal straw called a bombilla. Yerba mate is especially popular in Argentina, Brazil, Paraguay, and Uruguay and has enjoyed a long history in some cultures and is still often shared by passing the gourd among groups of people to show hospitality [1]. Mate’s economic importance is growing as products manufactured from mate and the tea itself are introduced worldwide. Growing popularity is partially due to the reputation of providing numerous health benefits, including increased energy and weight loss, as well as for treatment of many health problems from headaches to hypertension [2-7]. However, high incidence of esophageal cancer in populations with high mate tea consumption suggests a possible link between mate and cancer [8-20]. One important consideration is the relatively high levels of toxic PAHs in mate tea, likely due to processing with wood fires or other drying processes [8].
Polycyclic aromatic hydrocarbons are formed during combustion processes and are of concern because some are toxic to humans. Food is a common route of exposure for humans and some regulations exist for some foods and specific PAHs [21]. Historically, benzo[a]pyrene was used as the sole toxicity marker; however, data showed foods contain toxic PAHs without the presence of benzo[a]pyrene. The EFSA reevaluation suggested at least a subset of four PAHs, PAH4, as well as a subset of eight PAHs, PAH8, should be monitored in foods [21]. The PAH4 compounds are benzo[a]pyrene, chrysene, benz[a]anthracene, and benzo[b]fluoranthene. The PAH8 subset consists of the PAH4 plus benzo[k]fluoranthene, indeno[1,2,3-cd]pyrene, dibenz[ah]anthracene, and benzo[ghi]perylene. Development of analytical methods for PAHs should now focus on these subsets.
PAH analysis is challenging because there are isobaric PAHs that interfere with these PAHs of interest making accurate quantitation difficult, if not impossible. For example, chrysene is a toxic PAH and part of PAH4, but analysis of chrysene is complicated by the presence of triphenylene, which is an isobaric interference that completely or partially coelutes when using gas chromatography. This causes biasing of chrysene concentration or forces the compounds to be reported together. This is problematic because chrysene is toxic while triphenylene is not, often causing an overestimation of the toxicity in food items. Optimizing this separation and resolving other isobaric compounds is critical to providing correct quantitative data for the PAH4 and PAH8 compounds that are used as toxicity markers.
In addition, analysis of PAHs in foodstuffs is challenging because the compounds have to be determined at trace levels. Often, rigorous and time-consuming sample preparation is used to extract PAHs and clean up the sample before analysis. The complex nature of mate tea has led researchers to employ exhaustive sample preparation, including supercritical fluid extraction [22], pressurized fluid extraction [8], and gel permeation chromatography [23-25].
QuEChERS sample preparation methods are a desirable alternative because they are quick and easy, but still provide quality results. These methods typically work because they are paired with mass spectrometry based techniques like tandem mass spectrometry. Traditionally, QuEChERS involves a sample extraction followed by dispersive solid phase extraction cleanup [26, 27]. Although QuEChERS was originally designed for pesticide residues in fruit and vegetables [26], many modifications have been explored to expand the approach beyond the original scope. Compounds other than pesticides, like PAHs, veterinary drugs, and persistent organic pollutants, are now tested using QuEChERS approaches and difficult commodities like tea, spices, and tobacco have been tested using QuEChERS type methods [28-40].
This work describes the development of an analytical method for PAHs in tea that allows analysts to more quickly and accurately characterize target PAHs. Sample preparation is based on a modified QuEChERS extraction and solid phase extraction sample cleanup. Both GC-TOFMS and GC-MS/MS techniques were used and analyses were optimized for resolution of isobaric compounds, as well as for maintaining a reasonable analysis time.
Experimental
Materials
Development of this analytical method for PAHs in tea used six commercially available brands of dried yerba mate tea. All solvents were LC-MS grade or higher. The reference standards and sources were: EPA Method 8310 PAH mixture which contains 18 PAHs (cat.# 31874, Restek Corporation); 5-methylchrysene, benzo[c]phenanthrene, benzo[j]fluoranthene, cyclopenta[cd]pyrene, and dibenzo[a,e]pyrene (Cerilliant); coronene, dibenz[a,c]anthracene, perylene, and triphenylene (Sigma-Aldrich); benzo[e]pyrene (Ultra Scientific); and benzo(ghi)fluoranthene and benzo[a]fluoranthene (Santa Cruz Biotechnology). Although 30 PAHs were tested, special attention was paid to the PAH4* and PAH8 groups. The internal standard mix used was the SV internal standard mix (cat.# 31206, Restek Corporation). Original unbuffered QuEChERS extraction salts (cat.# 23992) and solid phase extraction cartridges with PTFE frits containing 500 mg silica (cat.# 28978) were also obtained from Restek Corporation.
*Since the completion of this work, Restek has developed an EFSA PAH4 certified reference material (cat.# 32469) prepared at 1,000 µg/mL in toluene that is both convenient and compatible with QuEChERS solvents.
Sample Preparation and Modified QuEChERS Extraction
Dried tea was powdered using a hand-held blender. QuEChERS extraction requires a sample with high water content (>80%). To prepare the dried tea material for a QuEChERS extraction, 1 g of powdered tea was combined with 10 mL of water in a FEP tube. After shaking to mix well, PAHs and internal standards were added. PAHs were fortified at 50 and 500 ng/g dry tea and internal standards were added at 100 ng/g. The sample was allowed to soak for 10 minutes and then 10 mL of hexane:acetone (1:1, v/v) were added. Samples were then vortexed for 30 minutes. The prepackaged unbuffered QuEChERS salts (4 g MgSO4 and 1 g NaCl) were added slowly. The samples were shaken by hand for 1 minute and then centrifuged for 5 minutes at 3,000 x g.
An investigation of extraction solvents was performed to determine whether using acetonitrile or hexane:acetone (1:1, v/v) produced better recoveries. The previously described procedure was used with some modifications. Two grams of powdered tea were fortified with 18 PAHs at 50 µg/g (in dry tea) using the EPA method 8310 PAH mixture (cat.# 31874). The PAH-fortified tea samples were soaked overnight at approximately 4 °C to maximize introduction of PAHs into the tea itself prior to their attempted extraction. The samples were then processed with the QuEChERS extraction and cleanup described in this work. The final optimized extraction procedure is summarized in Table I.
Solid Phase Extraction Cleanup
Two milliliters of extract was exchanged to hexane by evaporating to less than 1 mL using a gentle stream of nitrogen gas in a heating block at 50 °C, and then adding hexane for a total volume of 2 mL. This process was performed twice. The silica cartridge was conditioned with 3 mL of methanol followed by 3 mL of acetone under high vacuum, then with 3 mL of hexane:methylene chloride (1:1, v/v) and 6 mL of hexane at a rate of approximately 1 drop per second. One milliliter of extract was loaded onto the cartridge and eluted with 5 mL of various percentages of methylene chloride in hexane. Elution solvents tested were 0, 15, 25, 50, and 75 percent volume methylene chloride in hexane. Eluted samples were concentrated to 1 mL final volume with a gentle stream of nitrogen gas at 50 °C. The final cleanup procedure is summarized in Table I.
Table l: Final Extraction and Cleanup Procedure
| Modified QuEChERS Extraction |
| 1. Homogenize dry tea into a powder. |
| 2. Soak 1 g tea powder in 10 mL water for 10 min in an FEP centrifuge tube. |
| 3. Add 10 mL hexane:acetone (1:1) and vortex 30 min. |
| 4. Add Q-sep QuEChERS unbuffered salts (cat.# 23991), shake 1 min, and then spin for 5 min in a Q-sep 3000 centrifuge. |
| 5. Evaporate 2 mL of extract down to 1 mL, then adjust final volume to 2 mL with hexane. Perform this step twice. |
| Silica SPE Cleanup |
| 1. Rinse Resprep SPE cartridges (3 mL, 0.5 g silica; cat.# 28978) with 3 mL methanol followed by 3 mL acetone. |
| 2. Condition cartridges with 3 mL hexane:methylene chloride (1:1), followed by 6 mL hexane. |
| 3. Load 1 mL of extract onto cartridge and elute with 5 mL hexane:methylene chloride (7:3). |
| 4. Evaporate to 1 mL. |
Gas Chromatography Methods
Optimized chromatographic methods were used on three formats: GC-FID, GC-TOFMS, and GC-MS/MS. GC-FID was only used for extraction solvent evaluations. GC-TOFMS and GC-MS/MS platforms were used for spike recovery and quantitative analysis of incurred PAHs.
Gas Chromatograph-Flame Ionization Detection
GC-FID (GCxGC-FID instrument, LECO Corporation) was used to evaluate extraction solvents. A 30 m x 0.25 mm x 0.25 µm Rxi-5Sil MS column (cat.# 13623, Restek Corporation) was installed and operated with a constant flow of helium at 2 mL/min and an oven program of 80 °C, hold 0.1 min, then ramp at 8.5 °C/min to 330 °C and hold for 0.49 min. The inlet was held at 300 °C and outfitted with a Restek Premium 4.0 mm ID Precision inlet liner with wool (cat.# 23305, Restek Corporation). One microliter was injected using a split ratio of 10:1. The FID was held at 350 °C.
Gas Chromatography-Time of Flight Mass Spectrometry
A LECO Pegasus III GC-TOFMS instrument (LECO Corporation) was used for separation and quantification. Gas chromatography was performed using a high phenyl content Rxi-PAH GC column in a 60 m x 0.25 mm x 0.10 µm configuration (cat.# 49317, Restek Corporation). A splitless injection of 2.5 µL was performed using a Restek Premium 4 mm single taper inlet liner with wool (cat.# 23303, Restek Corporation). The inlet temperature was 275 °C. The splitless purge valve time was set to 1 min. A constant flow of hydrogen at 2.4 mL/min and oven temperature program of 80 °C (hold 1 min) ramping at 40 °C/min to 210 °C, then 3 °C/min to 260 °C, then 11.5 °C/min to 350 °C (hold 6.26 min) was used. The LECO Pegasus III TOFMS had a source temperature of 300 °C, used electron ionization at 70 eV, and stored a mass range of 45 to 550 u with an acquisition rate of 5 spectra/sec.
Gas Chromatography-Tandem Mass Spectrometry
GC-MS/MS analysis was performed using a Thermo TSQ 8000. (Thermo Fisher Scientific) equipped with a 40 m x 0.18 mm x 0.07 µm Rxi-PAH column (cat.# 49316, Restek Corporation), constant flow helium at 1.4 mL/min and a 2 mm single taper with wool inlet liner (cat.# 23316, Restek Corporation). The inlet was held at 275 °C and the oven program was 80 °C (hold 1 min), then 37 °C/min to 210 °C, then 3 °C/min to 260 °C, then 11 °C/min to 350 °C (hold 5.0 min). A splitless injection of 0.5 μL with a splitless time of 0.58 min and surge duration of 0.6 min was used. The transfer line was held at 330 °C. Three SRM transitions for each compound were collected. The SRM mode was not used in the typical manner where fragments are monitored. Instead, the SRM mode was used for the benefit of reduced background interferences. PAHs have a strong molecular ion, so transitions between the molecular ion, [M]+•, in Q1 and ions [M]+•, [M-H]+•, and [M-2H] +• in Q3 are used. Quantitation was based on one transition. The emission energy was 90 µA and the collision energy was set to 10.
Percent Recovery and Incurred PAH Determination
Fortified samples at 500 ng/g (ppb) were prepared with the optimized sample preparation procedure described in Table I. Acenaphthene-d10, chrysene-d12, naphthalene-d8, perylene-d12, and phenanthrene-d10 were used as internal standards. Internal standards were assigned to analytes by closest retention time to target analytes. Quantitation of fortified and unfortified samples was performed using a solvent calibration curve with levels of 50, 150, 500, 1,000 and 2,000 ppb.
Results and Discussion
Sample Extraction Solvent Investigation
QuEChERS is a desirable method for processing samples because it is quick, easy, and inexpensive. The initial QuEChERS approach used for this work started by hydrating the dry tea then extracting with acetonitrile followed by partitioning via the addition of salts. The hydration step is important for QuEChERS extractions because it is required for the proper partitioning to occur [41, 42]. Acetonitrile typically is used for QuEChERS extractions because it is an effective solvent for pesticides and can result in lower coextracted material for some matrices [41, 43]. However, solubility and recovery of PAHs with acetonitrile as the extraction solvent proved to be problematic. Based on the results of the extraction solvent investigation, hexane:acetone (1:1, v/v) is a stronger extraction solvent for PAHs and, thus, it was used instead of acetonitrile for the remaining experiments.
Table II shows recovery values for PAHs in mate tea samples fortified at 50 µg/g and processed with acetonitrile and hexane:acetone. There is a general trend of lower PAH recovery with acetonitrile compared to recovery using hexane:acetone (1:1, v/v). This is not unexpected as there is a polarity mismatch between PAHs, nonpolar compounds, and acetonitrile which is a polar solvent. Recovery values produced using acetonitrile also show a trend of progressively lower recovery for higher molecular weight PAHs. This can be attributed to the lower solubility of high molecular weight PAHs compared to lower molecular analogs. Recovery values of target PAHs with hexane:acetone (1:1, v/v) as the extraction solvent are in the acceptable range and no bias for high molecular weight PAHs is observed. Hexane:acetone (1:1, v/v) was used as the extraction solvent in all subsequent experiments.
Table ll: Percent recovery values for PAHs fortified at 50 µg/g in one yerba mate tea. Values shown compare acetonitrile and hexane:acetone (1:1, v/v) as the extraction solvents used during the initial sample extraction of wetted mate tea.
| PAH | Acetonitrile (% Recovery) |
Hexane:Acetone (1:1, v/v) % Recovery |
| Naphthalene | 73 | 91 |
| 2-Methylnaphthalene | 74 | 91 |
| 1-Methylnaphthalene | 73 | 99 |
| Acenaphthylene | 56 | 25 |
| Acenaphthene | 96 | 96 |
| Fluorene | 65 | 140 |
| Phenanthrene | 64 | 93 |
| Anthracene | 79 | 98 |
| Fluoranthene | 58 | 83 |
| Pyrene | 130 | 220 |
| Benz[a]anthracene | 55 | 89 |
| Chrysene | 55 | 80 |
| Benzo[b]fluoranthene | 53 | 110 |
| Benzo[k]fluoranthene | 55 | 140 |
| Benzo[a]pyrene | 51 | 83 |
| Indeno[1,2,3-cd]pyrene | 53 | 90 |
| Dibenz[a,h]anthracene | 56 | 98 |
| Benzo[ghi]perylene | 52 | 94 |
Evaluation of Methylene Chloride Percentage in Hexane Solvent for Sample Cleanup
Commodities like tea and spices can be particularly challenging because these matrices are very complex. Sample cleanup is needed to remove coextracted material to prepare samples for analysis. Extensive clean up techniques can greatly reduce the number and amount of coextracted compounds [44, 45]. However, these methods are often laborious, time-intensive processes.
The strategy for this work was to develop a simple cleanup process that removes enough coextracted material to allow for successful analysis, but also minimizes the resources needed to perform the cleanup. Gas chromatographic systems can be quickly fouled by introducing samples with a significant amount of nonvolatile material. This material resides in the GC inlet, dirtying the inlet liner and seals. The head of the column can also become contaminated with nonvolatile material that cannot be eluted from the column. This scenario necessitates instrument and column maintenance.
The silica SPE cleanup was optimized with respect to the elution solvent and volume. PAHs can be eluted with a combination of hexane and methylene chloride. Fortified tea samples at 50 µg/g were tested using different elution solvents with low to high methylene chloride percentages. Figure 1 displays the percent recovery values with respect to percent methylene chloride in hexane for low, mid, and high molecular weight PAHs. Acenaphthene is low molecular weight and was easily eluted with hexane; however, larger molecular weight compounds required a stronger solvent to elute from the silica. The plots of individual PAHs show increased recovery as the ratio of methylene chloride increases. At 15% methylene chloride in hexane, mid-sized PAHs, like the PAH4 shown in Figure 1, are recovered well with values around 100%. However, large PAHs like coronene are only recovered to approximately 60%. Based on the recovery values, a compromise of 30% methylene chloride in hexane was chosen for subsequent work. This elution solvent composition yielded acceptable recovery of all target PAHs. A higher ratio of methylene chloride would improve recovery of large PAHs, but would also increase the amount of coextracted material.
Figure 1: Percent recovery values of low, mid, and high molecular weight PAHs with respect to percent of methylene chloride in hexane used for silica SPE elution are plotted. PAHs were fortified at 50 µg/g.
Chromatographic Method
Optimizing the chromatographic separation is critical for PAH analysis due to the isobaric compounds that commonly coelute making quantitation difficult. An optimized GC method was developed using a high phenyl content Rxi-PAH column that is selective for the highly aromatic PAHs. Due to the selectivity of the column stationary phase and the column formats, analysis of the 30 PAHs used in the study, including the dibenzopyrenes, was accomplished in 35 minutes which is a relatively fast analysis time. Figure 2A shows an extracted ion chromatogram of m/z 226 and 228 for commercial tea 1, which was produced using the GC-TOFMS method described above. The separation of triphenylene and chrysene shows distinct peaks. This allowed for reliable peak integration for both compounds, which are isobaric congeners that usually elute at or near the same retention time. Similarly, benzo[b]fluoranthene, benzo[j]fluoranthene, and benzo[k]fluoranthene are notoriously challenging to separate, but these compounds also are well resolved as shown in Figure 2B. In total, the optimized method used for this work separated critical pairs and allowed accurate, independent quantitation of important toxicity markers including chrysene and benzo[b]fluoranthene which are EFSA PAH4 compounds and dibenz[a,h]anthracene which is a PAH8 compound.
Figure 2: A) GC-TOFMS extracted ion chromatogram of m/z 226 and 228 showing the separation of incurred PAHs, including triphenylene and chrysene, from commercial tea 1. B) GC-TOFMS extracted ion chromatogram of m/z 252 showing the separation of incurred benzofluoranthenes from commercial tea 1.
A
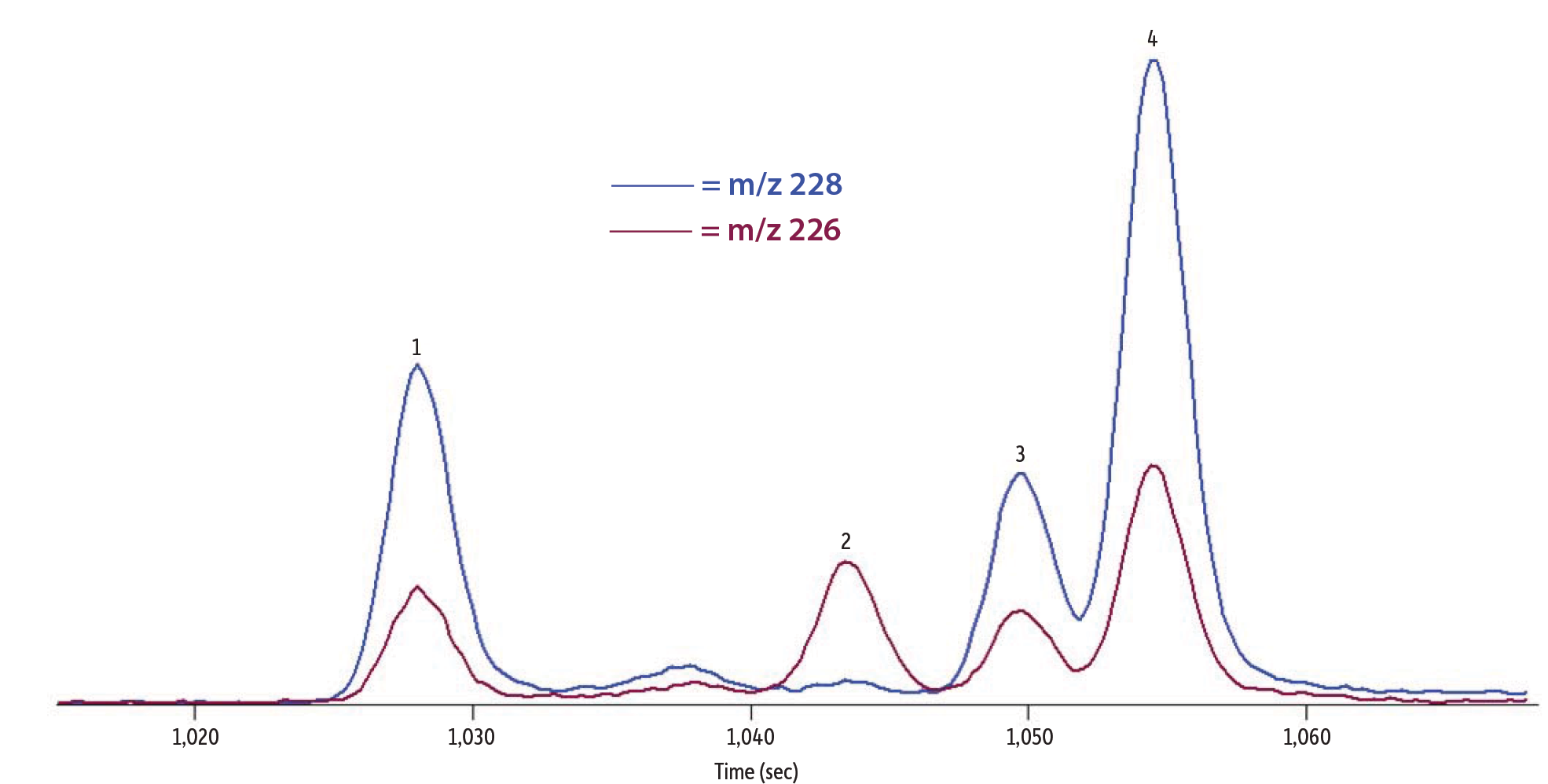
| Peaks | tR (sec) | |
|---|---|---|
| 1. | Benz[a]anthracene | 1,028.4 |
| 2. | Cyclopenta[cd]pyrene | 1,044.0 |
| 3. | Triphenylene | 1,050.0 |
| 4. | Chrysene | 1,054.8 |
B
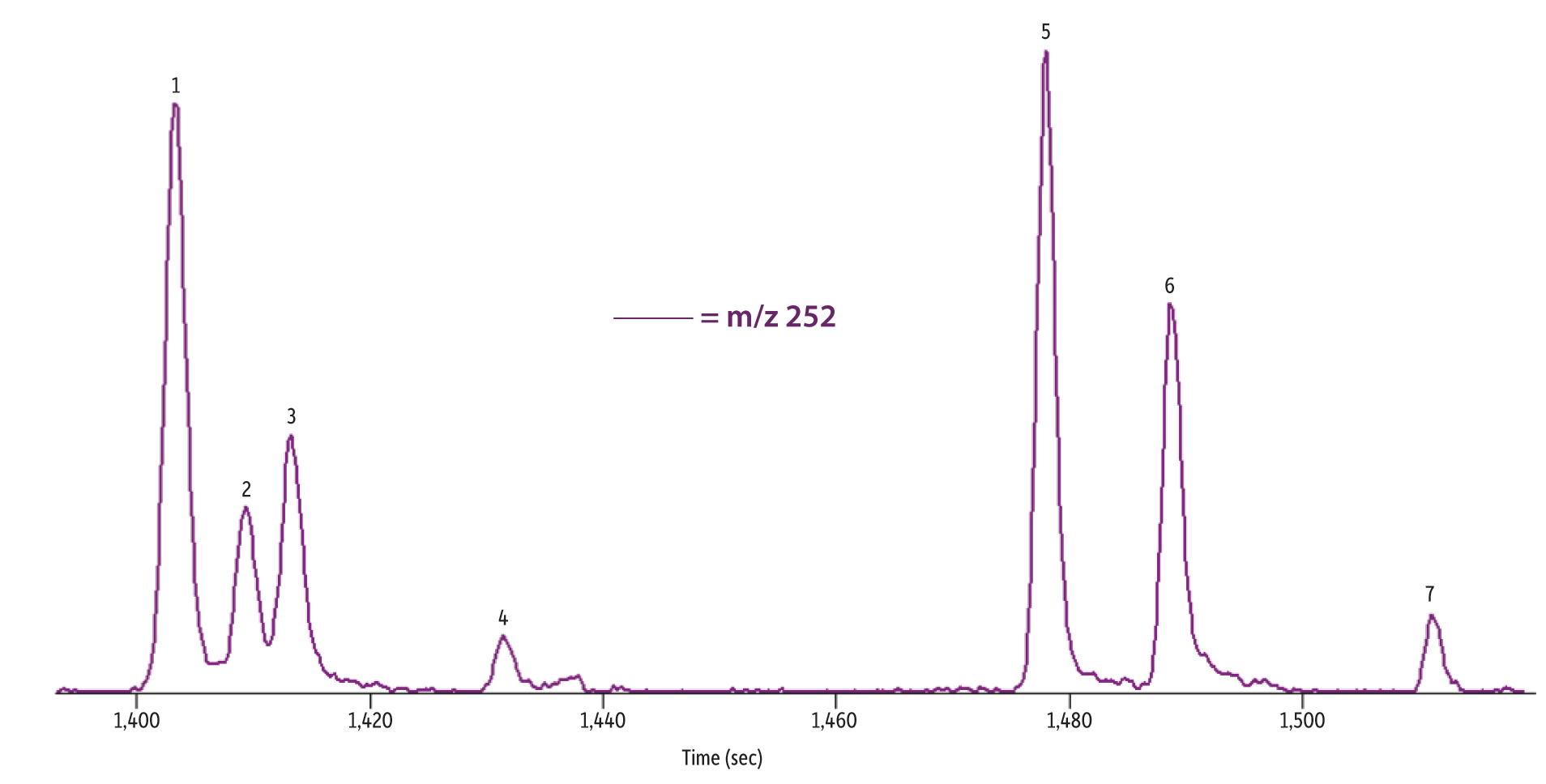
| Peaks | tR (sec) | |
|---|---|---|
| 1. | Benzo[b]fluoranthene | 1,403.6 |
| 2. | Benzo[k]fluoranthene | 1,409.6 |
| 3. | Benzo[j]fluoranthene | 1,413.6 |
| 4. | Benzo[a]fluoranthene | 1,431.6 |
| 5. | Benzo[e]pyrene | 1,478.2 |
| 6. | Benzo[a]pyrene | 1,489.0 |
| 7. | Perylene | 1,511.4 |
| Column | Rxi-PAH, 60 m, 0.25 mm ID, 0.10 µm (cat.# 49317) |
|---|---|
| Standard/Sample | |
| Injection | |
| Inj. Vol.: | 2.5 µL splitless (hold 1 min) |
| Liner: | Premium 4 mm single taper w/wool (cat.# 23303) |
| Inj. Temp.: | 275 °C |
| Purge Flow: | 40 mL/min |
| Oven | |
| Oven Temp.: | 80 °C (hold 1 min) to 210 °C at 40 °C/min to 260 °C at 3 °C/min to 350 °C at 11.5 °C/min (hold 6.25 min) |
| Carrier Gas | H2, constant flow |
| Flow Rate: | 2.4 mL/min |
| Detector | TOFMS |
|---|---|
| Transfer Line Temp.: | 320 °C |
| Analyzer Type: | TOF |
| Source Temp.: | 300 °C |
| Electron Energy: | 70 eV |
| Mass Defect: | 0 mu/100 u |
| Solvent Delay Time: | 3.67 min |
| Tune Type: | PFTBA |
| Ionization Mode: | EI |
| Acquisition Range: | 45-550 amu |
| Spectral Acquisition Rate: | 5 spectra/sec |
| Instrument | LECO Pegasus 4D GCxGC-TOFMS |
Incurred PAHs in Teas
Quantitative analysis was easily accomplished using this optimized chromatographic method. Incurred values of isobaric compounds were evaluated in six commercial mate teas and results are displayed in Table III. Quantitative bias is demonstrated by comparing the concentrations of compounds in each isobaric pair, which are grouped between grey rows. Based on values in Table III, chrysene would be biased about 20%, benzo[b]fluoranthene by approximately 50%, and dibenzo[a,h]anthracene by as much as 60% if the combined area of the pairs had to be reported. The ability to separate these compounds is critical to determining the presence and concentration of toxicity marker PAHs.
Table III Values of incurred PAHs in six different mate teas determined using the final extraction/cleanup method and GC-TOFMS. Isobaric pairs are grouped and separated by a grey-colored row in the table.
|
PAH |
Tea 1 |
Tea 2 |
Tea 3 |
Tea 4 |
Tea 5 |
Tea 6 |
|
Triphenylene |
82 |
14 |
18 |
54 |
34 |
14 |
|
Chrysene |
320 |
81 |
85 |
260 |
140 |
130 |
|
Benzo[b]fluoranthene |
150 |
67 |
35 |
150 |
49 |
52 |
|
Benzo[j]fluoranthene |
65 |
39 |
20 |
75 |
27 |
31 |
|
Dibenz[a,c]anthracene |
11 |
9.3 |
10 |
12 |
10 |
6.9 |
|
Dibenz[a,h]anthracene |
18 |
15 |
10 |
21 |
12 |
12 |
Recovery of PAHs in Fortified Teas
The optimized method used a modified QuEChERS extraction with hexane:acetone (1:1, v/v), silica SPE cleanup, and a selective Rxi-PAH GC column. Both GC-MS/MS and GC-TOFMS were able to perform the method and yielded satisfactory recovery values, but GC-MS/MS offered better sensitivity. Recovery values are shown in Table IV. The recovery values for all PAHs in this study range between 72 and 130% with only four compounds in the 70 to 80% range. Recoveries for the EFSA PAH4 compounds were 81-100% and recoveries for the EFSA PAH8 compounds were 81-110%. The recovery values indicate that this analytical method for PAHs in tea is suitable for PAHs with a wide range of volatility and molecular weight.
Table IV: Percent recovery values for PAHs in a commercially available tea. PAHs were fortified at 500 µg/g dry tea.
|
PAH |
Tea 1 |
|
Naphthalene |
90 |
|
Acenaphthylene |
110 |
|
Acenaphthene |
99 |
|
Fluorene |
110 |
|
Phenanthrene |
81 |
|
Anthracene |
130 |
|
Fluoranthene |
72 |
|
Pyrene |
74 |
|
Benzo[c]phenanthrene |
75 |
|
Benz[a]anthracene |
81 |
|
Triphenylene |
80 |
|
Chrysene |
82 |
|
5-Methylchrysene |
76 |
|
Benzo[b]fluoranthene |
92 |
|
Benzo[k]fluoranthene |
96 |
|
Benzo[j]fluoranthene |
89 |
|
Benzo[a]fluoranthene |
97 |
|
Benzo[e]pyrene |
89 |
|
Benzo[a]pyrene |
100 |
|
Perylene |
94 |
|
Dibenz[a,c]anthracene |
100 |
|
Indeno[1,2,3-cd]pyrene |
110 |
|
Dibenz[a,h]anthracene |
98 |
|
Benzo[ghi]perylene |
88 |
|
Dibenzo[a,e]pyrene |
93 |
|
Coronene |
86 |
Comparison of GC-TOFMS and GC-MS/MS for EFSA PAH4 Compounds
Samples of six yerba mate teas were processed with the optimized sample preparation method and two GC-MS based methods. The GC-TOFMS method used a selective Rxi-PAH GC column in a 60 m x 0.25 mm x 0.10 µm configuration (cat.# 49317) format that increases the separation of isobaric compounds as well as sample loading capacity. Peak resolution was enhanced by use of hydrogen carrier gas. PAHs are ideal for analysis by hydrogen carrier GC-MS because they form strong molecular ions and do not suffer from hydrogen reactivity, thus mitigating potential sensitivity loss when using hydrogen in GC-MS. The GC-MS/MS also used the selective Rxi-PAH GC column, but in a 40 m x 0.18 mm x 0.07 µm format (cat.# 49316) that balances separation with analysis time. In addition, sample loading is smaller because the stationary phase film thickness is relatively thin. Thus, it was important to minimize the sample injection volume for this method. Mate tea samples had relatively high incurred PAHs concentrations, so using a 0.5 µL injection was not detrimental to overall detectability. However, for other commodities with trace levels of PAHs, using a highly sensitive tandem MS can compensate for lower injection volumes.
The concentrations of incurred PAH4 compounds were determined by both GC-TOFMS and GC-MS/MS and are shown in Table V. The combined levels for the PAH4 are shown in the last row. The values determined by both techniques agree well with each other. This indicates that both the sample preparation and analysis methods are suitable for PAH analysis in mate tea.
Table V: Incurred concentrations, ng/g dry tea, for the PAH4 compounds in six brands of yerba mate tea. For each tea, values are reported for the GC-MS/MS and GC-TOFMS methods. The combined concentrations of the PAH4 are reported in the last row.
|
PAH |
Tea 1 |
Tea 2 |
Tea 3 |
Tea 4 |
Tea 5 |
Tea 6 |
||||||
|
MS/MS |
TOF |
MS/MS |
TOF |
MS/MS |
TOF |
MS/MS |
TOF |
MS/MS |
TOF |
MS/MS |
TOF |
|
|
Benz[a]anthracene |
190 |
190 |
33 |
45 |
43 |
52 |
150 |
170 |
94 |
100 |
52 |
62 |
|
Chrysene |
320 |
320 |
46 |
81 |
70 |
85 |
250 |
260 |
140 |
140 |
110 |
130 |
|
Benzo[b]fluoranthene |
150 |
150 |
78 |
67 |
57 |
35 |
140 |
150 |
70 |
49 |
79 |
52 |
|
Benzo[a]pyrene |
120 |
140 |
66 |
82 |
24 |
36 |
160 |
160 |
42 |
42 |
80 |
81 |
|
Combined EFSA PAH4 |
780 |
800 |
220 |
270 |
190 |
210 |
700 |
740 |
350 |
340 |
320 |
320 |
Conclusion
The mate teas tested have high levels of PAHs when compared to typical residue limits of between 1 and 10 ng/g. The EFSA PAH4 sums are shown in the last row of Table V and range from 190-800 ng/g in dry tea. The streamlined sample preparation method for PAHs in yerba mate tea provided satisfactory recovery of all PAHs tested. The selective chromatographic methods were paired with MS-based detection. Sufficient separation of isobaric PAHs was accomplished using Rxi-PAH columns with a PAH selective stationary phase making quantitation of individual PAHs straightforward. Overall, this analytical method for PAHs in tea required less resources and time than typically needed for analysis of a difficult matrix like mate tea while providing improved data quality.
References
- Guayakí. Mate Gourd Ceremony. http://guayaki.com/mate/2611/Mate-Gourd-Ceremony.html (Accessed June 28, 2013).
- D. H. M. Bastos, D. M. d. Oliveira, R. L. T. Matsumoto, P. d. O. Carvalho, M. L. Ribeiro, Yerba maté: Pharmacological Properties, Research and Biotechnology, Medicinal and Aromatic Plant Science and Biotechnology 1 (2007) 37.
- R. L. Matsumoto, S. Mendonca, D. M. de Oliveira, M. F. Souza, D. H. Bastos, Effects of mate tea intake on ex vivo LDL peroxidation induced by three different pathways, Nutrients 1 (2009) 18.
- M. Bixby, L. Spieler, T. Menini, A. Gugliucci, Ilex paraguariensis extracts are potent inhibitors of nitrosative stress: a comparative study with green tea and wines using a protein nitration model and mammalian cell cytotoxicity, Life Sci. 77 (2005) 345.
- H. Gao, Y. Long, X. Jiang, Z. Liu, D. Wang, Y. Zhao, D. Li, B. L. Sun, Beneficial effects of Yerba Mate tea (Ilex paraguariensis) on hyperlipidemia in high-fat-fed hamsters, Exp. Gerontol. 48 (2013) 572.
- F. Martins, A. J. Suzan, S. M. Cerutti, D. P. Arcari, M. L. Ribeiro, D. H. Bastos, O. Carvalho Pde, Consumption of mate tea (Ilex paraguariensis) decreases the oxidation of unsaturated fatty acids in mouse liver, Br. J. Nutr. 101 (2009) 527.
- P. E. Resende, S. G. Verza, S. Kaiser, L. F. Gomes, L. C. Kucharski, G. G. Ortega, The activity of mate saponins (Ilex paraguariensis) in intra-abdominal and epididymal fat, and glucose oxidation in male Wistar rats, J. Ethnopharmacol. 144 (2012) 735.
- F. Kamangar, M. M. Schantz, C. C. Abnet, R. B. Fagundes, S. M. Dawsey, High levels of carcinogenic polycyclic aromatic hydrocarbons in mate drinks, Cancer Epidemiol. Biomarkers Prev. 17 (2008) 1262.
- V. Sewram, E. D. Stefani, P. Brennan, P. Boffetta, Maté Consumption and the Risk of Squamous Cell Esophageal Cancer in Uruguay, Cancer Epidemiology, Biomarkers & Prevention 12 (2003) 508.
- M. N. Bates, C. Hopenhayn, O. A. Rey, L. E. Moore, Bladder cancer and mate consumption in Argentina: a case-control study, Cancer Lett. 246 (2007) 268.
- R. Castelletto, X. Castellsague, N. Munoz, J. Iscovich, N. Chopita, A. Jmelnitsky, Alcohol, tobacco, diet, mate drinking, and esophageal cancer in Argentina, Cancer Epidemiol. Biomarkers Prev. 3 (1994) 557.
- X. Castellsague, N. Munoz, E. De Stefani, C. G. Victora, R. Castelletto, P. A. Rolon, Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America, Int. J. Cancer 88 (2000) 658.
- A. P. Dasanayake, A. J. Silverman, S. Warnakulasuriya, Mate drinking and oral and oro-pharyngeal cancer: a systematic review and meta-analysis, Oral Oncol. 46 (2010) 82.
- E. De Stefani, P. Correa, L. Fierro, E. Fontham, V. Chen, D. Zavala, Black tobacco, mate, and bladder cancer. A case-control study from Uruguay, Cancer 67 (1991) 536.
- E. De Stefani, L. Fierro, P. Correa, E. Fontham, A. Ronco, M. Larrinaga, J. Balbi, M. Mendilaharsu, Mate drinking and risk of lung cancer in males: a case-control study from Uruguay, Cancer Epidemiol. Biomarkers Prev., 5 (1996) 515.
- E. De Stefani, L. Fierro, M. Mendilaharsu, A. Ronco, M. T. Larrinaga, J. C. Balbi, S. Alonso, H. Deneo-Pellegrini, Meat intake, 'mate' drinking and renal cell cancer in Uruguay: a case-control study, Br. J. Cancer 78 (1998) 1239.
- D. Goldenberg, Mate: a risk factor for oral and oropharyngeal cancer, Oral Oncol. 38 (2002) 646.
- D. Loria, E. Barrios, R. Zanetti, Cancer and yerba mate consumption: a review of possible associations, Rev Panam Salud Publica 25 (2009) 530.
- P. A. Rolon, X. Castellsague, M. Benz, N. Munoz, Hot and cold mate drinking and esophageal cancer in Paraguay, Cancer Epidemiol. Biomarkers Prev. 4 (1995) 595.
- V. Sewram, E. De Stefani, P. Brennan, P. Boffetta, Mate consumption and the risk of squamous cell esophageal cancer in uruguay, Cancer Epidemiol. Biomarkers Prev. 12 (2003) 508.
- Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food, The EFSA Journal 724 (2008) 1.
- S. Schlemitz, W. Pfannhauser, Supercritical fluid extraction of mononitrated polycyclic aromatic hydrocarbons from tea – correlation with the PAH concentration, Zeitschrift für Lebensmitteluntersuchung und -Forschung A 205 (1997) 305.
- M. Ciecierska, M. W. Obiedzinski, Polycyclic aromatic hydrocarbons in the bakery chain, Food Chem. 141 (2013) 1.
- B. Dusek, J. Hajslova, V. Kocourek, Determination of nitrated polycyclic aromatic hydrocarbons and their precursors in biotic matrices, J. Chromatogr. A, 982 (2002) 127.
- W. Jira, K. Ziegenhals, K. Speer, Gas chromatography-mass spectrometry (GC-MS) method for the determination of 16 European priority polycyclic aromatic hydrocarbons in smoked meat products and edible oils, Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 25 (2008) 704.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher, F. J. Schenck, Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce, J. AOAC International 83 (2003)
- S. J. Lehotay, K. Mastovska, A. R. Lightfield, Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables, J. AOAC Int. 88 (2005) 615.
- P. Yogendrarajah, C. Van Poucke, B. De Meulenaer, S. De Saeger, Development and validation of a QuEChERS based liquid chromatography tandem mass spectrometry method for the determination of multiple mycotoxins in spices, J. Chromatogr. A 1297 (2013) 1.
- H. Yan, X. Liu, F. Cui, H. Yun, J. Li, S. Ding, D. Yang, Z. Zhang, Determination of amantadine and rimantadine in chicken muscle by QuEChERS pretreatment method and UHPLC coupled with LTQ Orbitrap mass spectrometry, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 938C (2013) 8.
- I. M. Valente, C. M. Santos, M. M. Moreira, J. A. Rodrigues, New application of the QuEChERS methodology for the determination of volatile phenols in beverages by liquid chromatography, J. Chromatogr. A 1271 (2013) 27.
- S. Shoeibi, M. Amirahmadi, H. Rastegar, R. Khosrokhavar, A. M. Khaneghah, An applicable strategy for improvement recovery in simultaneous analysis of 20 pesticides residue in tea, J. Food Sci. 78 (2013) T792.
- A. Sadowska-Rociek, M. Surma, E. Cieslik, Application of QuEChERS method for simultaneous determination of pesticide residues and PAHs in fresh herbs, Bull. Environ. Contam. Toxicol. 90 (2013) 508.
- W. Peysson, E. Vulliet, Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography-time-of-flight-mass spectrometry, J. Chromatogr. A, 1290 (2013) 46.
- F. Lega, L. Contiero, G. Biancotto, R. Angeletti, Determination of thyreostats in muscle and thyroid tissues by QuEChERS extraction and ultra-performance liquid chromatography tandem mass spectrometry, Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. (2013)
- V. Homem, J. Avelino Silva, C. Cunha, A. Alves, L. Santos, New analytical method for the determination of musks in personal care products by Quick, Easy, Cheap, Effective, Rugged, and Safe extraction followed by GC-MS, J. Sep. Sci. (2013)
- A. Albinet, S. Tomaz, F. Lestremau, A really quick easy cheap effective rugged and safe (QuEChERS) extraction procedure for the analysis of particle-bound PAHs in ambient air and emission samples, Sci. Total Environ. 450-451 (2013) 31.
- M. Amirahmadi, S. Shoeibi, M. Abdollahi, H. Rastegar, R. Khosrokhavar, M. P. Hamedani, Monitoring of some pesticides residue in consumed tea in Tehran market, Iranian J. Environ. Health Sci. Eng. 10 (2013) 9.
- K. Usui, Y. Hayashizaki, M. Hashiyada, M. Funayama, Rapid drug extraction from human whole blood using a modified QuEChERS extraction method, Leg. Med. (Tokyo) 14 (2012) 286.
- J. W. Shi, Y. G. Zhao, Z. J. Fu, J. G. Li, Y. F. Wang, T. C. Yang, Development of a screening method for the determination of PCBs in water using QuEChERS extraction and gas chromatography-triple quadrupole mass spectrometry, Anal. Sci. 28 (2012) 167.
- K. Mastovska, K. J. Dorweiler, S. J. Lehotay, J. S. Wegscheid, K. A. Szpylka, Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques, J. Agric. Food Chem. 58 (2010) 5959.
- I. R. Pizzutti, A. de Kok, M. Hiemstra, C. Wickert, O. D. Prestes, Method validation and comparison of acetonitrile and acetone extraction for the analysis of 169 pesticides in soya grain by liquid chromatography-tandem mass spectrometry, J. Chromatogr. A, 1216 (2009) 4539.
- K. Maštovská, S. J. Lehotay, Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues, J. Chromatogr. A 1040 (2004) 259.
- Z. Huang, Y. Li, B. Chen, S. Yao, Simultaneous determination of 102 pesticide residues in Chinese teas by gas chromatography-mass spectrometry, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 853 (2007) 154.
- G. F. Pang, Y. M. Liu, C. L. Fan, J. J. Zhang, Y. Z. Cao, X. M. Li, Z. Y. Li, Y. P. Wu, T. T. Guo, Simultaneous determination of 405 pesticide residues in grain by accelerated solvent extraction then gas chromatography-mass spectrometry or liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem. 384 (2006) 1366.

