Fast Analysis of Δ8-THC, Δ9-THC, and Isomeric Hydroxy and Carboxy Metabolites in Whole Blood by LC-MS/MS
Introduction
The testing of whole blood samples for tetrahydrocannabinol (Δ9-THC) consumption is routine and has been performed for many decades. Since Δ9-THC is metabolized into 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-Δ9-THC) and further into 11-nor-9-carboxy-Δ9-THC (Δ9-THC-COOH), it is important to test for the parent compound and both metabolites to properly monitor for THC usage.
As more isomers of Δ9-THC become available on the market, testing has become more complicated, and novel methods are needed to achieve isomeric resolution. One such isomer, Δ8-THC, is federally unregulated in the United States and readily available for purchase in many stores. This compound forms its own hydroxylated and carboxylated metabolites, (11-OH-Δ8-THC and Δ8-THC-COOH), that must be resolved from their isomeric Δ9 counterparts. Chromatographic resolution of all three isomeric pairs is essential for reporting accurate clinical specimen results, and poor resolution, especially when one isomer is present in much greater abundance than the other, can cause invalid data.
To improve reporting accuracy, the following method was developed to adequately resolve all six isomers in whole blood. Linearity, accuracy, precision, and the potential for cross-analyte interferences were assessed.
Experimental
Liquid-Liquid Extraction [1]
- 500 µL aliquots of blank whole blood were added to glass test tubes.
- 50 µL of calibrator or QC standard was added to each tube. 50 µL of internal standard (1000 ng/mL of Δ9-THC-D3; 11-OH-Δ9-THC-D3; and Δ9-THC-COOH-D3) was added to each tube and vortexed.
- 500 µL of HPLC grade water was added to each tube and vortexed.
- 100 µL of 1N HCl was added to each tube and vortexed.
- 2.5 mL of 80:20 hexanes:ethyl acetate was added to each tube, capped, and vortexed until visibly combined.
- Samples were centrifuged at 4200 rpm for 15 minutes or until the two layers had completely separated.
- The supernatant was pipetted off each sample, transferred to a clean test tube, and dried down under nitrogen.
- Samples were reconstituted in 100 µL of 50:50 water:methanol (both containing 0.1% formic acid), vortexed, and transferred to an LC vial with an insert.
Instrument Parameters
The LC-MS/MS method detailed in Tables I and II was developed on a Raptor FluoroPhenyl column in order to optimally separate Δ8-THC; Δ9-THC; 11-OH-Δ8-THC; 11-OH-Δ9-THC; Δ8-THC-COOH; and Δ9-THC-COOH. MRM transitions and ESI mode for each analyte are given in Figure 1.
Table I: Chromatography Gradient
| Time (min) | Flow Rate (mL/min) | %A | %B |
| 0.00 | 0.8 | 36 | 64 |
| 6.50 | 0.8 | 36 | 64 |
| 6.60 | 0.8 | 32 | 68 |
| 13.00 | 0.8 | 32 | 68 |
| 13.10 | 0.8 | 0 | 100 |
| 14.00 | 0.8 | 0 | 100 |
| 14.10 | 0.8 | 36 | 64 |
| 16.00 | 0.8 | 36 | 64 |
Table II: LC Method Parameters
| Column | Raptor FluoroPhenyl, 2.7 μm, 100 mm x 3.0 mm ID (cat.# 9319A1E) |
| Guard | Raptor FluoroPhenyl EXP guard column cartridge 5 mm, 3.0 mm ID, 2.7 μm (cat.# 9319A0253) |
| Column Temperature | 40 °C |
| Mobile Phase A | 0.1% formic acid in water |
| Mobile Phase B | 0.1% formic acid in methanol |
| Injection Volume | 10 µL |
Results & Discussion
Chromatographic Performance
As shown in Figure 1, all three sets of isomers (six total analytes) were well separated in a fast 13-minute gradient (16-minute total analysis time) on a Raptor FluoroPhenyl column. The FluoroPhenyl stationary phase provided better selectivity for all three isomer pairs compared to alternative column chemistries, such as biphenyl or C18.
Figure 1: Separation of Δ8/9-THC, Hydroxy, and Carboxy Metabolites in Whole Blood
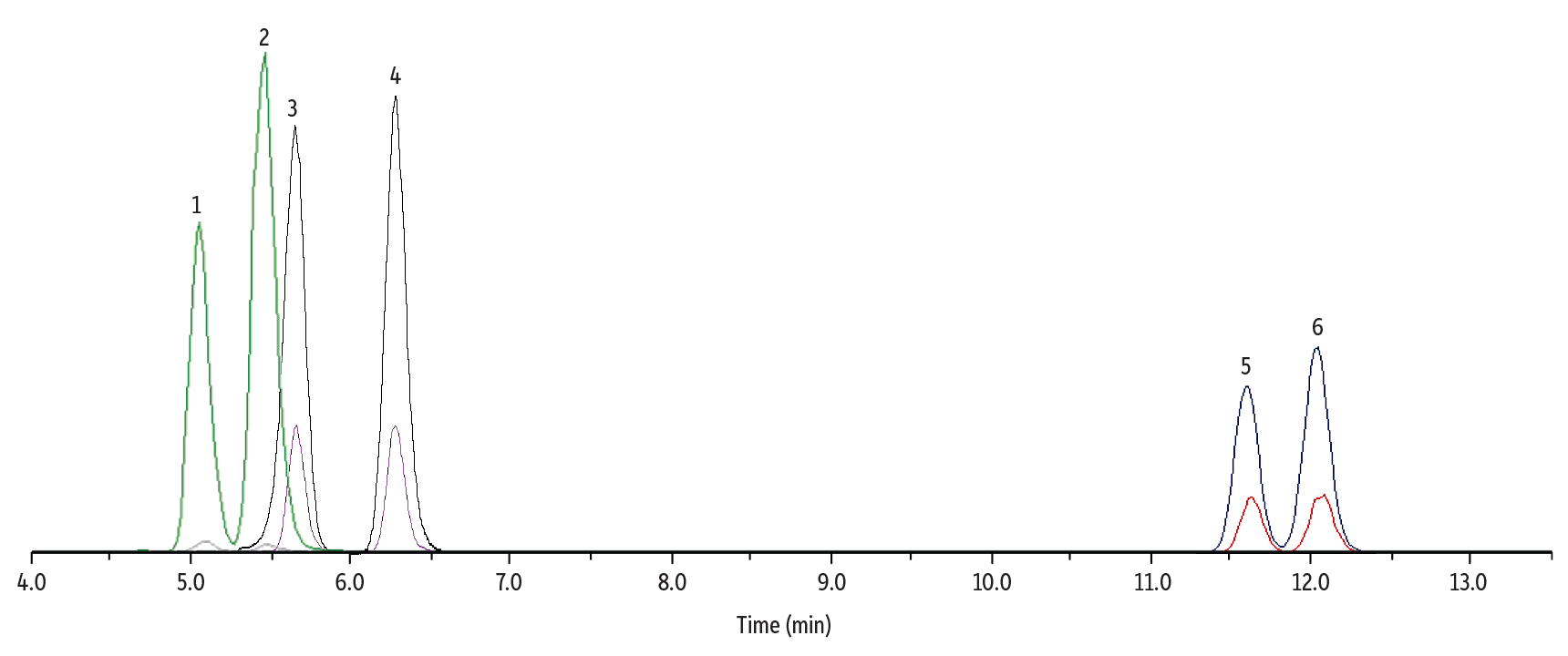
| Peaks | tR (min) | Conc. (ng/mL) | Precursor | Product 1 | Product 2 | Mode | |
|---|---|---|---|---|---|---|---|
| 1. | 11-OH-Δ8-THC | 5.10 | 10 | 331.4 | 313.0 | 193.2 | + |
| 2. | 11-OH-Δ9-THC | 5.49 | 10 | 331.4 | 313.0 | 193.2 | + |
| 3. | Δ8-THC-COOH | 5.54 | 50 | 343.0 | 298.9 | 244.8 | - |
| 4. | Δ9-THC-COOH | 6.45 | 50 | 343.0 | 298.9 | 244.8 | - |
| 5. | Δ8-THC | 11.67 | 10 | 315.5 | 193.0 | 123.0 | + |
| 6. | Δ9-THC | 12.18 | 10 | 315.5 | 193.0 | 123.0 | + |
| Column | Raptor FluoroPhenyl (cat.# 9319A1E) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 3.0 mm ID | ||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor FluoroPhenyl EXP guard column cartridge 5 mm, 3.0 mm ID, 2.7 µm (cat.# 9319A0253) | ||||||||||||||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||||||||||||||
| Standard/Sample | Δ8-Tetrahydrocannabinol (Δ8-THC) (cat.# 34090) | ||||||||||||||||||||||||||||||||||||
| Δ9-Tetrahydrocannabinol (Δ9-THC) (cat.# 34067) | |||||||||||||||||||||||||||||||||||||
| (±)11-nor-9-carboxy-Δ-9-THC (Δ9-THC-COOH) (cat.# 34068) | |||||||||||||||||||||||||||||||||||||
| Other compounds obtained separately. | |||||||||||||||||||||||||||||||||||||
| Diluent: | 50:50 Methanol:water, both with 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Max Pressure: | 440 bar |
| Detector | SCIEX 4500 MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+/ESI- |
| Sample Preparation | 500 µL of whole blood was transferred to a 15 mL glass test tube. 50 µL of internal standard and 50 µL of control material were transferred to the test tube and vortexed. 500 µL of HPLC grade water was added to each sample and vortexed. 100 µL of 1N HCl was added to each sample and vortexed. 2.5 mL of 80:20 hexanes:ethyl acetate was added to each sample and vortexed until visibly combined. Samples were centrifuged at 4200 rpm for 15 minutes. The top layer was transferred to a new glass test tube and dried down under nitrogen. Samples were reconstituted with 100 µL of 50:50 methanol:water, both containing 0.1% formic acid, and vortexed. Samples were transferred to 2 mL screw-thread vials (cat.# 21143) with glass inserts (cat.# 21776) and capped with short-cap, screw-vial closures (cat.# 24498). |
Linearity, Accuracy, and Precision
Linearity was demonstrated using a 1/x2 weighted linear regression, and all analytes showed acceptable R2 values (≥0.99). The linear calibration ranges were 0.5–100 ng/mL for 11-OH-Δ8-THC; 11-OH-Δ9-THC; Δ8-THC; and Δ9-THC and 2.5–500 ng/mL for Δ8-THC-COOH and Δ9-THC-COOH.
Accuracy and precision were assessed on three different days using low, mid, and high QC samples spiked at 5, 10, and 50 ng/mL for 11-OH-Δ8-THC; 11-OH-Δ9-THC; Δ8-THC; and Δ9-THC and at 25, 50, and 250 ng/mL for Δ8-THC-COOH and Δ9-THC-COOH. Method accuracy was demonstrated by recovery values being within 10% of the nominal concentrations for the QC samples at all levels. The %RSD was under 20% for both intraday and interday testing, indicating acceptable method precision.
Cross-Analyte Interferences
To demonstrate that other naturally occurring cannabinoids would not interfere with the target compounds, we spiked whole blood with nine commonly encountered and/or structurally similar cannabinoids: CBDV, CBD, CBG, THCV, exo-THC, CBL, CBN, Δ10-THC, and CBC. All nine cannabinoids were fully resolved from the analytes of interest on the Raptor FluoroPhenyl column and no cross-analyte interferences are expected (Figure 2).
Figure 2: Separation of Δ8/9-THC, Hydroxy, and Carboxy Metabolites from Nine Cannabinoids
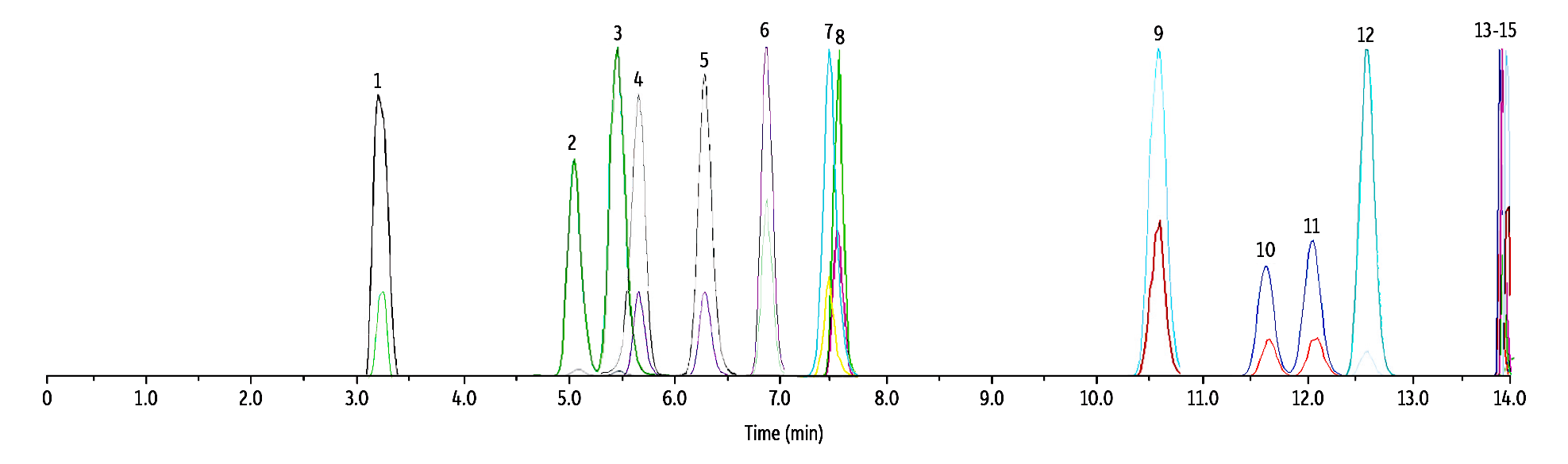
| Peaks | tR (min) | Conc. (ng/mL) | Precursor | Product 1 | Product 2 | Mode | |
|---|---|---|---|---|---|---|---|
| 1. | CBDV | 3.52 | 50 | 287.4 | 165.0 | 231.1 | + |
| 2. | 11-OH-Δ8-THC | 5.09 | 50 | 331.4 | 313.0 | 193.2 | + |
| 3. | 11-OH-Δ9-THC | 5.50 | 50 | 331.4 | 313.0 | 193.2 | + |
| 4. | Δ8-THC-COOH | 5.53 | 250 | 343.0 | 298.9 | 244.8 | - |
| 5. | Δ9-THC-COOH | 6.44 | 250 | 343.0 | 298.9 | 244.8 | - |
| 6. | CBD | 6.81 | 50 | 315.5 | 193.0 | 259.0 | + |
| 7. | CBG | 7.56 | 50 | 317.5 | 193.0 | 123.0 | + |
| 8. | THCV | 7.65 | 50 | 287.4 | 165.0 | 123.0 | + |
| 9. | exo-THC | 10.63 | 50 | 315.5 | 193.0 | 259.0 | + |
| 10. | Δ8-THC | 11.67 | 50 | 315.5 | 193.0 | 123.0 | + |
| 11. | Δ9-THC | 12.17 | 50 | 315.5 | 193.0 | 123.0 | + |
| 12. | CBL | 12.43 | 50 | 315.5 | 235.2 | 193.0 | + |
| 13. | Δ10-THC | 13.88 | 50 | 315.5 | 193.1 | 259.0 | + |
| 14. | CBN | 13.93 | 50 | 311.4 | 223.0 | 293.1 | + |
| 15. | CBC | 13.93 | 50 | 315.5 | 193.1 | 259.0 | + |
| Column | Raptor FluoroPhenyl (cat.# 9319A1E) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 3.0 mm ID | ||||||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||||||
| Guard Column: | Raptor FluoroPhenyl EXP guard column cartridge 5 mm, 3.0 mm ID, 2.7 µm (cat.# 9319A0253) | ||||||||||||||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||||||||||||||
| Standard/Sample | Cannabinoids Neutrals 9 standard (cat.# 34132) | ||||||||||||||||||||||||||||||||||||
| Δ8-Tetrahydrocannabinol (Δ8-THC) (cat.# 34090) | |||||||||||||||||||||||||||||||||||||
| Δ9-Tetrahydrocannabinol (Δ9-THC) (cat.# 34067) | |||||||||||||||||||||||||||||||||||||
| (±)11-nor-9-carboxy-Δ-9-THC (Δ9-THC-COOH) (cat.# 34068) | |||||||||||||||||||||||||||||||||||||
| Other compounds obtained separately. | |||||||||||||||||||||||||||||||||||||
| Diluent: | 50:50 Methanol:water, both with 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Max Pressure: | 440 bar |
| Detector | SCIEX 4500 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+/ESI- |
| Sample Preparation | 500 µL of whole blood was transferred to a 15 mL glass test tube. 50 µL of internal standard and 50 µL of control material were transferred to the test tube and vortexed. 500 µL of HPLC grade water was added to each sample and vortexed. 100 µL of 1N HCl was added to each sample and vortexed. 2.5 mL of 80:20 hexanes:ethyl acetate was added to each sample and vortexed until visibly combined. Samples were centrifuged at 4200 rpm for 15 minutes. The top layer was transferred to a new glass test tube and dried down under nitrogen. Samples were reconstituted with 100 µL of 50:50 methanol:water, both containing 0.1% formic acid, and vortexed. Samples were transferred to 2 mL screw-thread vials (cat.# 21143) with glass inserts (cat.# 21776) and capped with short-cap, screw-vial closures (cat.# 24498). |
Conclusion
The fast LC-MS/MS method established here on a Raptor FluoroPhenyl column provides essential chromatographic resolution of Δ8-THC and Δ9-THC isomers as well as their isomeric hydroxy and carboxy metabolites in whole blood. In addition, the target compounds were completely separated from potentially interfering cannabinoids, helping ensure that labs can report more accurate clinical results.
References
N.B. Tiscione, R . Miller, X . Shan, J . Sprague, D.T. Yeatman, An efficient, robust method for the determination of cannabinoids in whole blood by LC-MS-MS, J. Ana.l Toxicol. 40(8) (2016) 639-648. https://doi.org/10.1093/jat/bkw063

