High-Resolution GC Analyses of Fatty Acid Methyl Esters (FAMEs)
Fatty acid methyl esters (FAMEs) analysis is an important tool both for characterizing fats and oils and for determining the total fat content in foods. Fats can be extracted from a matrix using a nonpolar solvent and saponified to produce salts of the free fatty acids. After derivatizing the free acids to form methyl esters, the mixture can readily be analyzed by gas chromatography (GC) due to the volatility and thermal stability of the FAMEs. Gas chromatography has become an important technique in fats and oils analysis because accurate results can be obtained for complex as well as simple sample matrices.
FAMEs analyses were among the first applications for gas chromatography, so many of the GC methods originally written for analysis of fats and oils described packed column technology. However, capillary columns offer significant advantages, including more efficient separations. When analyzing fats and oils with complex fatty acid profiles such as the cis and trans forms of polyunsaturated fatty acids, higher efficiencies are needed to resolve the individual components. Capillary columns with Carbowax-type (polyethylene glycol) stationary phases are typically used for analyses of saturated and unsaturated fatty acid methyl esters (Figures 1 and 2), and biscyanopropyl phases are used to resolve cis and trans isomers of polyunsaturated components (Figure 3).
Creating FAMEs
Lipids are normally extracted from matrices using a nonpolar solvent such as ether and saponified to produce the free fatty acid salts. The fatty acid salts then are derivatized to form the fatty acid methyl esters, to increase volatility, improve peak symmetry, and decrease sample activity, thus providing more accurate analytical data. The official methods of the Association of Official Agriculture Chemists (AOAC International) [1] and the American Oil Chemists Society (AOCS) [2] contain procedures for the derivatization reaction, as does the European Pharmacopoeia [3]. In general, the glycerides are saponified by refluxing with methanolic sodium hydroxide. The esterification is effected with a reagent such as boron trifluoride in methanol, and the FAMEs are extracted with a nonpolar solvent (e.g., heptane) for analysis by GC.
Several groups of researchers have proposed simplified procedures for creating the methyl esters. For example, lipids can be transmethylated in situ. This option combines all of the conventional steps, except the drying and post-reaction workup, into one step. [4] For some samples, trimethylsulfonium hydroxide (TMSH), sodium methoxide, or methanolic hydrochloric acid, which are alternative derivatization reagents, can be used for transesterification. A major advantage of this approach is that the derivatization can be performed in a single, fast reaction step. [5,6,7]
Figure 1: A FAMEWAX column provides fast, efficient separations of FAMEs in a marine oil-based standard.
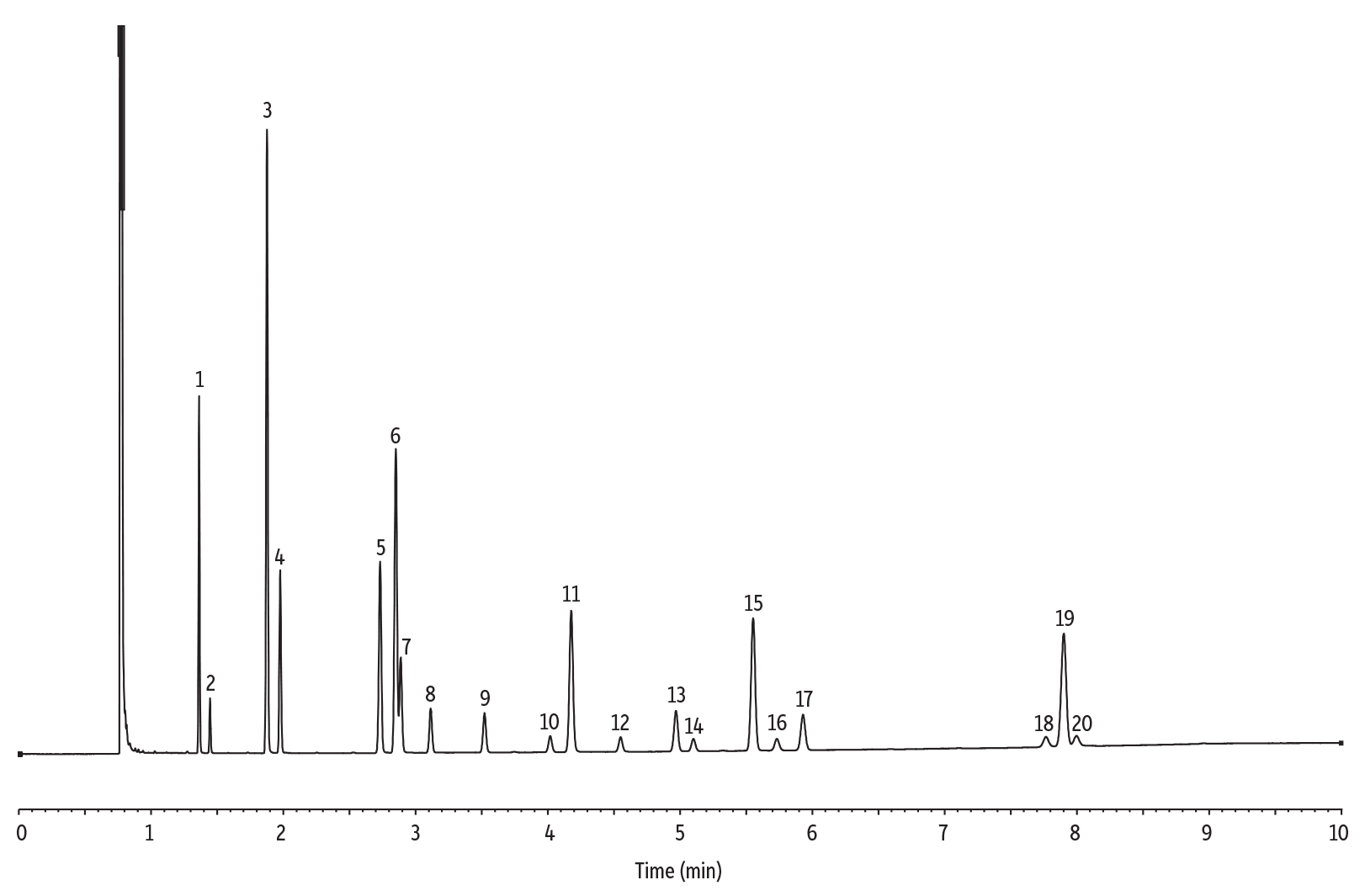
| Peaks | |
|---|---|
| 1. | C14:0 |
| 2. | C14:1 |
| 3. | C16:0 |
| 4. | C16:1 |
| 5. | C18:0 |
| 6. | C18:1 (oleate) |
| 7. | C18:1 (vaccenate) |
| 8. | C18:2 n6 c |
| 9. | C18:3 n3 |
| 10. | C20:0 |
| Peaks | |
|---|---|
| 11. | C20:1 n9 |
| 12. | C20:2 n6 |
| 13. | C20:4 n6 |
| 14. | C20:3 n3 |
| 15. | C20:5 n3 |
| 16. | C22:0 |
| 17. | C22:1 n9 |
| 18. | C24:0 |
| 19. | C22:6 n3 |
| 20. | C24:1 |
| Column | FAMEWAX, 30 m, 0.32 mm ID, 0.25 µm (cat.# 12498) |
|---|---|
| Standard/Sample | Marine oil FAME mix (cat.# 35066) |
| Conc.: | 10 mg/mL total |
| Injection | |
| Inj. Vol.: | 0.5 µL split (split ratio 150:1) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 195 °C to 240 °C at 5 °C/min (hold 1 min) |
| Carrier Gas | H2, constant flow |
| Linear Velocity: | 62 cm/sec |
| Detector | FID @ 250 °C |
|---|---|
| Notes | Liner cat.# 20936-202.1 was used to produce this chromatogram, but has since been discontinued. For assistance choosing a replacement for this application, contact Restek Technical Service or your local Restek representative. |
Figure 2: Rapid, excellent resolution of ocean nutrition sample FAMEs, using a FAMEWAX column.
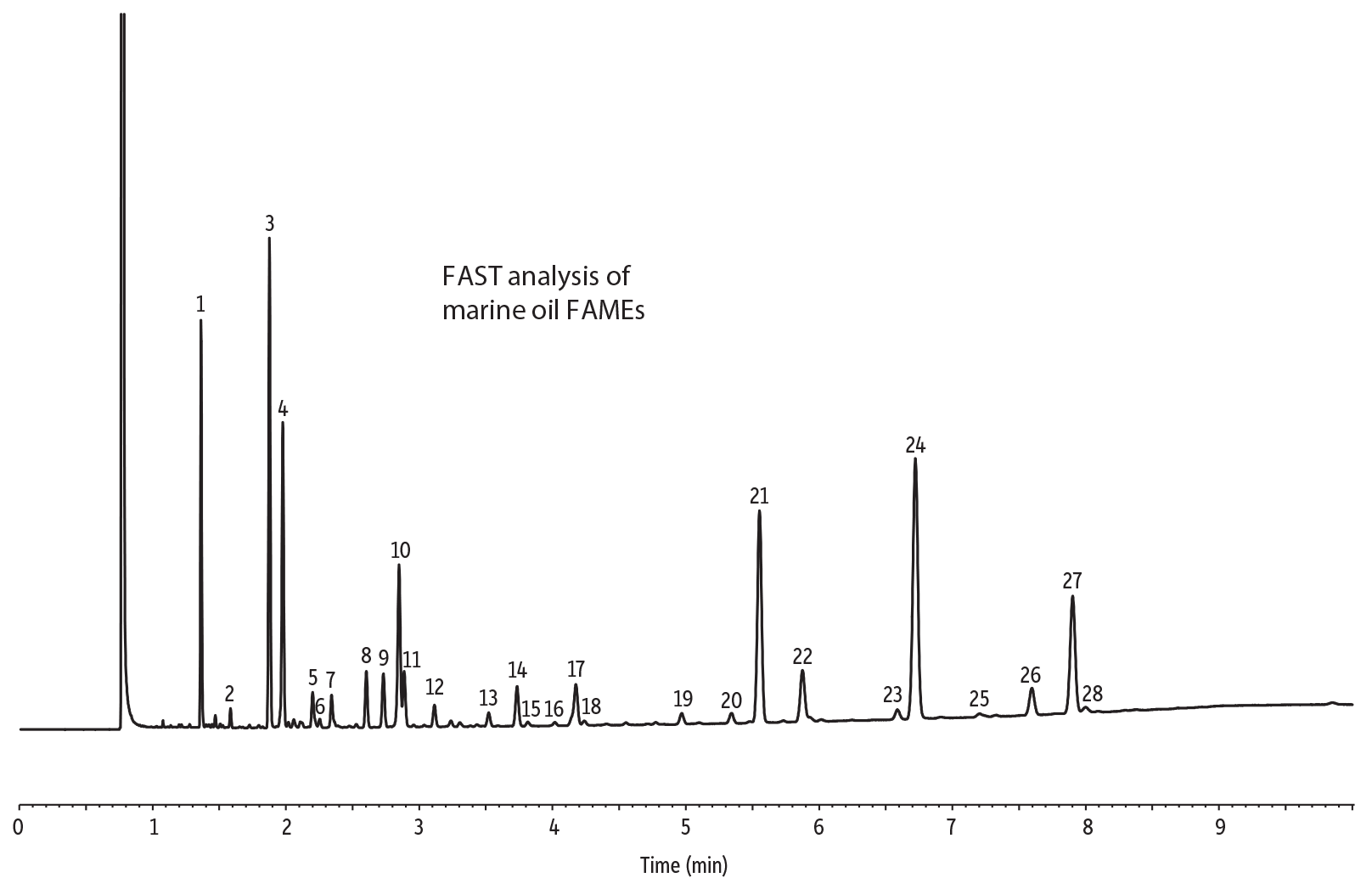
| Peaks | |
|---|---|
| 1. | C14:0 |
| 2. | C15:0 |
| 3. | C16:0 |
| 4. | C16:1 |
| 5. | C16:2 |
| 6. | C17:0 |
| 7. | C17:1 |
| 8. | C16:4 |
| 9. | C18:0 |
| 10. | C18:1 (oleate) |
| 11. | C18:1 (vaccenate) |
| 12. | C18:2 n6 cis |
| 13. | C18:3 n3 |
| 14. | C18:4 n6 |
| Peaks | |
|---|---|
| 15. | C18:4 n3 |
| 16. | C20:0 |
| 17. | C20:1 n7 |
| 18. | C20:1 n9 |
| 19. | C20:4 n6 |
| 20. | C20:4 n3 |
| 21. | C20:5 n3 |
| 22. | C22:1 n7 |
| 23. | C21:5 n3 |
| 24. | C23:0 (IS) |
| 25. | C22:5 n6 |
| 26. | C22:5 n3 |
| 27. | C22:6 n3 |
| 28. | C24:1 |
| Column | FAMEWAX, 30 m, 0.32 mm ID, 0.25 µm (cat.# 12498) |
|---|---|
| Standard/Sample | Ocean nutrition sample |
| Conc.: | 12 mg/mL total FAMEs |
| Injection | |
| Inj. Vol.: | 0.5 µL split (split ratio 150:1) |
| Liner: | 3 mm split w/ wool |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 195 °C to 240 °C at 5 °C/min (hold 1 min) |
| Carrier Gas | H2, constant flow |
| Linear Velocity: | 62 cm/sec |
| Detector | FID @ 250 °C |
|---|
Figure 3: Near-complete resolution of a 37 FAME mix on an Rt-2560 column.
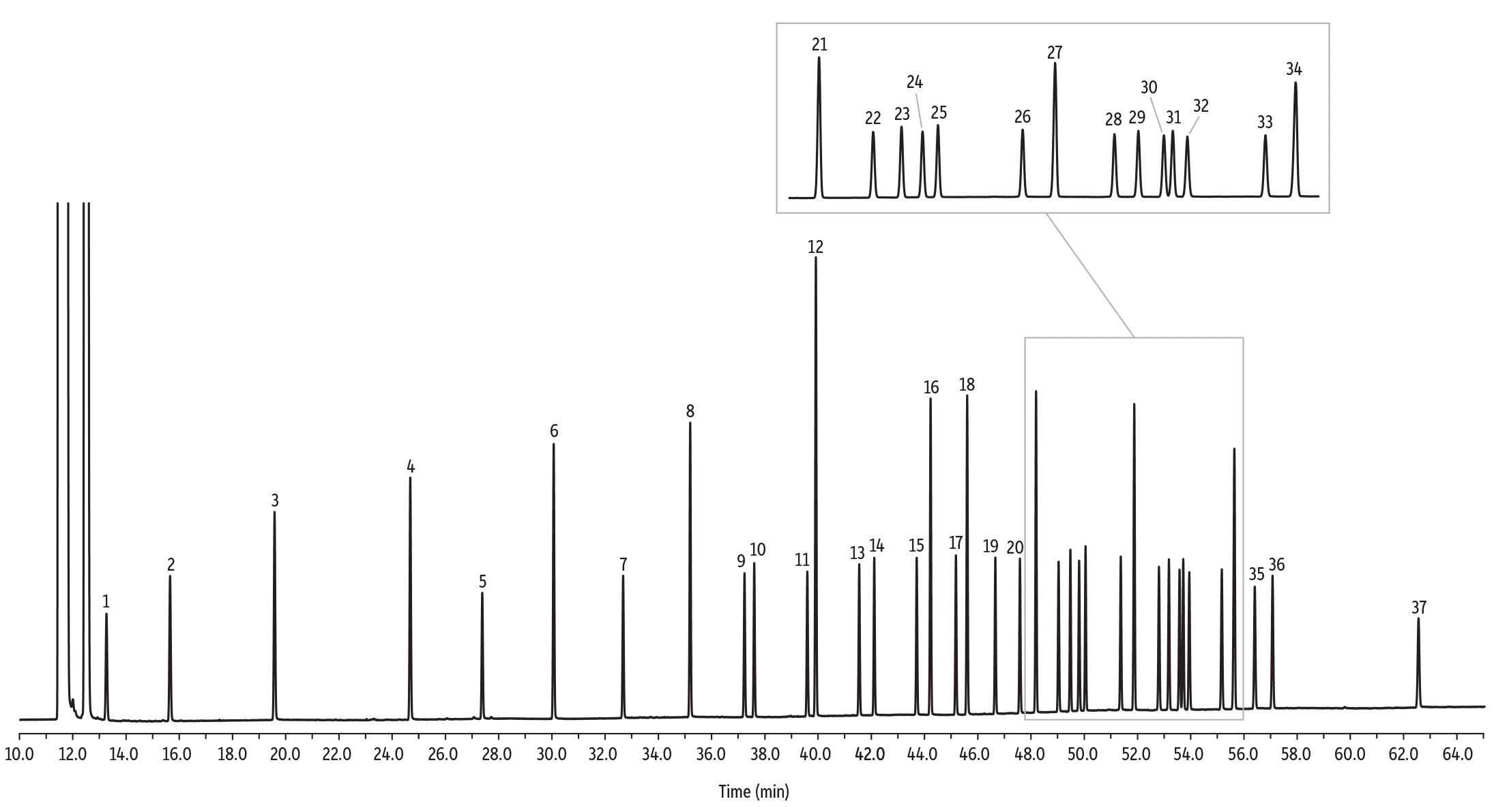
| Peaks | tR (min) | Conc. (mg/mL) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl butyrate | 13.16 | 40 | C4:0 |
| 2. | Methyl caproate | 15.53 | 40 | C6:0 |
| 3. | Methyl octanoate | 19.43 | 40 | C8:0 |
| 4. | Methyl decanoate | 24.50 | 40 | C10:0 |
| 5. | Methyl undecanoate | 27.20 | 20 | C11:0 |
| 6. | Methyl dodecanoate | 29.87 | 40 | C12:0 |
| 7. | Methyl tridecanoate | 32.47 | 20 | C13:0 |
| 8. | Methyl myristate | 34.97 | 40 | C14:0 |
| 9. | Methyl myristoleate | 37.01 | 20 | C14:1 (c9) |
| 10. | Methyl pentadecanoate | 37.37 | 20 | C15:0 |
| 11. | Methyl pentadecenoate | 39.36 | 20 | C15:1 (c10) |
| 12. | Methyl palmitate | 39.68 | 60 | C16:0 |
| 13. | Methyl palmitoleate | 41.30 | 20 | C16:1 (c9) |
| 14. | Methyl heptadecanoate | 41.86 | 20 | C17:0 |
| 15. | Methyl heptadecenoate | 43.45 | 20 | C17:1 (c10) |
| 16. | Methyl stearate | 43.97 | 40 | C18:0 |
| 17. | Methyl octadecenoate | 44.92 | 20 | C18:1 (t9) |
| 18. | Methyl oleate | 45.34 | 40 | C18:1 (c9) |
| Peaks | tR (min) | Conc. (mg/mL) | Structural Nomenclature | |
|---|---|---|---|---|
| 19. | Methyl linolelaidate | 46.39 | 20 | C18:2 (t9,t12) |
| 20. | Methyl linoleate | 47.32 | 20 | C18:2 (c9,c12) |
| 21. | Methyl arachidate | 47.92 | 40 | C20:0 |
| 22. | Methyl linolenate | 48.77 | 20 | C18:3 (c6,c9,c12) |
| 23. | Methyl eicosenoate | 49.20 | 20 | C20:1 (c11) |
| 24. | Methyl linolenate | 49.54 | 20 | C18:3 (c9,c12,c15) |
| 25. | Methyl heneicosanoate | 49.78 | 20 | C21:0 |
| 26. | Methyl eicosadienoate | 51.09 | 20 | C20:2 (c11,c14) |
| 27. | Methyl behenate | 51.60 | 40 | C22:0 |
| 28. | Methyl eicosatrienoate | 52.52 | 20 | C20:3 (c8,c11,c14) |
| 29. | Methyl erucate | 52.88 | 20 | C22:1 (c13) |
| 30. | Methyl eicosatrienoate | 53.28 | 20 | C20:3 (c11,c14,c17) |
| 31. | Methyl arachidonate | 53.42 | 20 | C20:4 (c5,c8,c11,c14) |
| 32. | Methyl tricosanoate | 53.65 | 20 | C23:0 |
| 33. | Methyl docosadienoate | 54.85 | 20 | C22:2 (c13,c16) |
| 34. | Methyl lignocerate | 55.32 | 40 | C24:0 |
| 35. | Methyl eicosapentaenoate | 56.09 | 20 | C20:5 (c5,c8,c11,c14,c17) |
| 36. | Methyl nervonate | 56.74 | 20 | C24:1 (C15) |
| 37. | Methyl docosahexaenoate | 62.17 | 20 | C22:6 (c4,c7,c10,c13,c16,c19) |
| Column | Rt-2560, 100 m, 0.25 mm ID, 0.20 µm (cat.# 13198) |
|---|---|
| Standard/Sample | Food industry FAME mix (cat.# 35077) |
| Diluent: | Hexane/dichloromethane |
| Conc.: | 1,000 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 20:1) |
| Liner: | Premium 4 mm Precision liner w/wool (cat.# 23305) |
| Inj. Temp.: | 225 °C |
| Oven | |
| Oven Temp.: | 100 °C (hold 4 min) to 240 °C at 3 °C/min (hold 15 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.0 mL/min |
| Detector | FID @ 285 °C |
|---|---|
| Make-up Gas Flow Rate: | 45 mL/min |
| Make-up Gas Type: | N2 |
| Hydrogen flow: | 30 mL/min |
| Air flow: | 300 mL/min |
| Data Rate: | 20 Hz |
| Instrument | Agilent 7890A GC |
Analyzing Polyunsaturated FAMEs
The FAMEWAX polyethylene glycol stationary phase is specially tested with a polyunsaturated FAMEs mix to ensure resolution of the omega-3 and omega-6 fatty acids of interest. FAMEs such as methyl eicosapentenoate (C20:5) and methyl docosahexaenoate (C22:6), found in nutraceutical ingredients and products such as marine oils, also are resolved. FAMEWAX columns offer excellent resolution of polyunsaturated FAMEs with significantly reduced analysis times compared to traditional Carbowax stationary phases. In fact, analysis times of less than 10 minutes are possible! Figures 1 and 2 show analyses of a marine oil FAME standard and ocean nutrition sample, respectively. Both analyses are characterized by fast, effective resolution and sharp, symmetric peaks.
Like FAMEWAX columns, Stabilwax columns and Rtx-Wax columns provide excellent resolution of FAMEs derived from either plant or animal sources. When polyunsaturated FAMEs are analyzed on one of these Carbowax-type capillary columns, analysis times of 35-50 minutes are generally required to fully resolve the C21:5 FAME from the C23:0 internal standard, and the C24:0 FAME from C22:6.
Resolving cis and trans Isomers
Individual cis and trans isomers are resolved on a 100-meter Rt-2560 column, making this the column of choice for analyzing partially hydrogenated fats. The highly polar biscyanopropyl phase gives the selectivity needed for resolving FAME isomers, such as the cis and trans forms of C18:1. The trans isomers elute before the cis isomers on this phase, opposite of the elution order on Carbowax-based phases such as FAMEWAX or Rtx-Wax. Figure 3 shows the chromatographic separation of 37 FAMEs typically encountered in vegetable, animal, or marine fats and oils using an Rt-2560 column.
AOAC method 996.06 [1] describes the determination of total fat content based on the fatty acid content after conversion to methyl esters. This is the specified method for determining total fat content for nutritional labeling purposes. After quantifying the total FAMEs present in the derivatized sample, the amount of fat (as triglycerides) in the sample is calculated based on initial sample weight. The 100-meter Rt-2560 column meets the requirements of this procedure (Figure 4). This column also allows quantification of the total trans content.
Figure 4: Use this food industry FAME mix to standardize fat-by-fatty acid composition methods, such as AOAC 996.06.
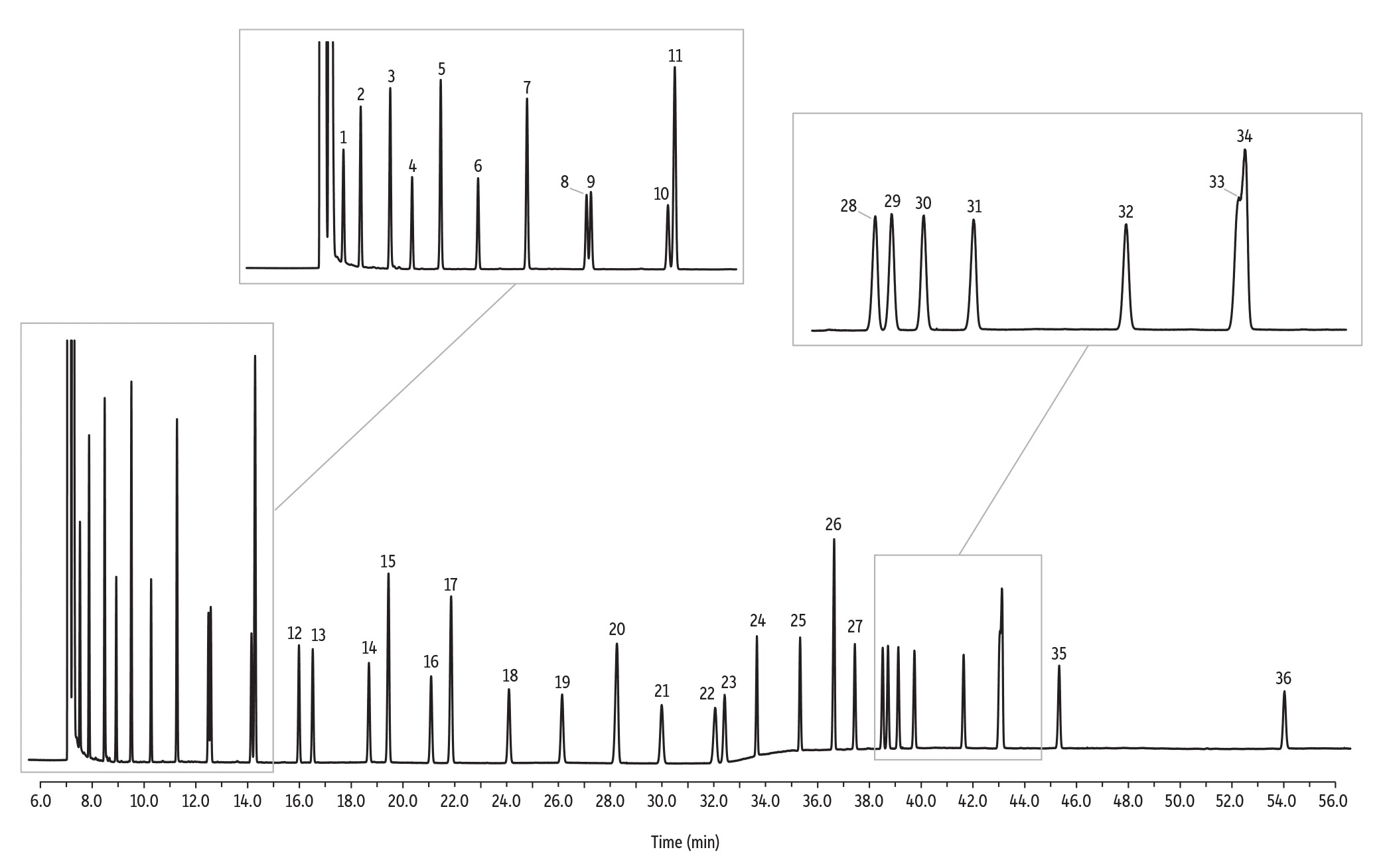
| Peaks | tR (min) | Conc. (mg/mL) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl caproate | 7.52 | 40 | C6:0 |
| 2. | Methyl octanoate | 7.88 | 40 | C8:0 |
| 3. | Methyl decanoate | 8.48 | 40 | C10:0 |
| 4. | Methyl undecanoate | 8.93 | 20 | C11:0 |
| 5. | Methyl dodecanoate | 9.51 | 40 | C12:0 |
| 6. | Methyl tridecanoate | 10.27 | 20 | C13:0 |
| 7. | Methyl myristate | 11.27 | 40 | C14:0 |
| 8. | Methyl myristoleate | 12.48 | 20 | C14:1 (c9) |
| 9. | Methyl pentadecanoate | 12.57 | 20 | C15:0 |
| 10. | Methyl pentadecenoate | 14.15 | 20 | C15:1 (C10) |
| 11. | Methyl palmitate | 14.28 | 60 | C16:0 |
| 12. | Methyl palmitoleate | 15.98 | 20 | C16:1 (c9) |
| 13. | Methyl heptadecanoate | 16.51 | 20 | C17:0 |
| 14. | Methyl heptadecenoate | 18.68 | 20 | C17:1 (c10) |
| 15. | Methyl stearate | 19.43 | 40 | C18:0 |
| 16. | Methyl octadecenoate | 21.08 | 20 | C18:1 (t9) |
| 17. | Methyl oleate | 21.85 | 40 | C18:1 (c9) |
| 18. | Methyl linolelaidate | 24.09 | 20 | C18:2 (t9,t12) |
| Peaks | tR (min) | Conc. (mg/mL) | Structural Nomenclature | |
|---|---|---|---|---|
| 19. | Methyl linoleate | 26.14 | 20 | C18:2 (c9,c12) |
| 20. | Methyl arachidate | 28.25 | 40 | C20:0 |
| 21. | Methyl linolenate | 29.98 | 20 | C18:3 (c6,c9,c12) |
| 22. | Methyl eicosenoate | 32.05 | 20 | C20:1 (c11) |
| 23. | Methyl linolenate | 32.41 | 20 | C18:3 (c9,c12,c15) |
| 24. | Methyl heneicosanoate | 33.66 | 20 | C21:0 |
| 25. | Methyl eicosadienoate | 35.33 | 20 | C20:2 (c11,c14) |
| 26. | Methyl behenate | 36.64 | 40 | C22:0 |
| 27. | Methyl eicosatrienoate | 37.44 | 20 | C20:3 (c8,c11,c14) |
| 28. | Methyl erucate | 38.51 | 20 | C22:1 (c13) |
| 29. | Methyl eicosatrienoate | 38.72 | 20 | C20:3 (c11,c14,c17) |
| 30. | Methyl arachidonate | 39.12 | 20 | C20:4 (c5,c8,c11,c14) |
| 31. | Methyl tricosanoate | 39.74 | 20 | C23:0 |
| 32. | Methyl docosadienoate | 41.64 | 20 | C22:2 (c13,c16) |
| 33. | Methyl eicosapentaenoate | 43.07 | 20 | C20:5 (c5,c8,c11,c14,c17) |
| 34. | Methyl lignocerate | 43.11 | 40 | C24:0 |
| 35. | Methyl nervonate | 45.33 | 20 | C24:1 (c15) |
| 36. | Methyl docosahexaenoate | 54.02 | 20 | C22:6 (c4,c7,c10,c13,c16,c19) |
| Column | Rt-2560, 100 m, 0.25 mm ID, 0.20 µm (cat.# 13198) |
|---|---|
| Standard/Sample | Food industry FAME mix (cat.# 35077) |
| Diluent: | Hexane/dichloromethane |
| Conc.: | 1,000 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 20:1) |
| Liner: | Premium 4 mm Precision liner w/wool (cat.# 23305) |
| Inj. Temp.: | 235 °C |
| Oven | |
| Oven Temp.: | 180 °C (hold 32 min) to 215 °C at 20 °C/min (hold 31.25 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Detector | FID @ 325 °C |
|---|---|
| Make-up Gas Flow Rate: | 45 mL/min |
| Make-up Gas Type: | N2 |
| Hydrogen flow: | 30 mL/min |
| Air flow: | 300 mL/min |
| Data Rate: | 20 Hz |
| Instrument | Agilent 7890A GC |
| Notes | C4:0 Methyl butyrate (623-42-7) elutes in the solvent front. |
To calibrate the GC system for assays of this type, use a FAME mixture such as our 37-component food industry FAME mix (Figures 3 and 4), or our 28-component food industry FAME mix. These standards include a gravimetric certificate of analysis to help ensure accurate quantification. To ensure correct identifications of the individual cis and trans isomers, use our cis/trans FAMEs mix as shown in Figure 5, or our trans fat mix shown in Figure 6.
Rtx-2330, a 90% biscyanopropyl phase, also resolves cis and trans FAME isomers. These columns are slightly less polar than Rt-2560 columns. Figure 7 shows the analysis of an animal-based fat using an Rtx-2330 column. As on Rt-2560 columns, the trans forms of the FAMEs elute before the cis forms.
Analyzing Botanical Products
Gas chromatography can be used to analyze fatty acid marker compounds in some botanical products. Both Rtx-Wax and Stabilwax capillary columns provide the efficiency and selectivity needed to perform analysis and allow accurate identification of the individual fatty acids in products such as saw palmetto oil, olive oil, and palm oil (Figures 8-10).
Figure 5: Resolve cis and trans isomers of unsaturated FAMEs on an Rt-2560 column.
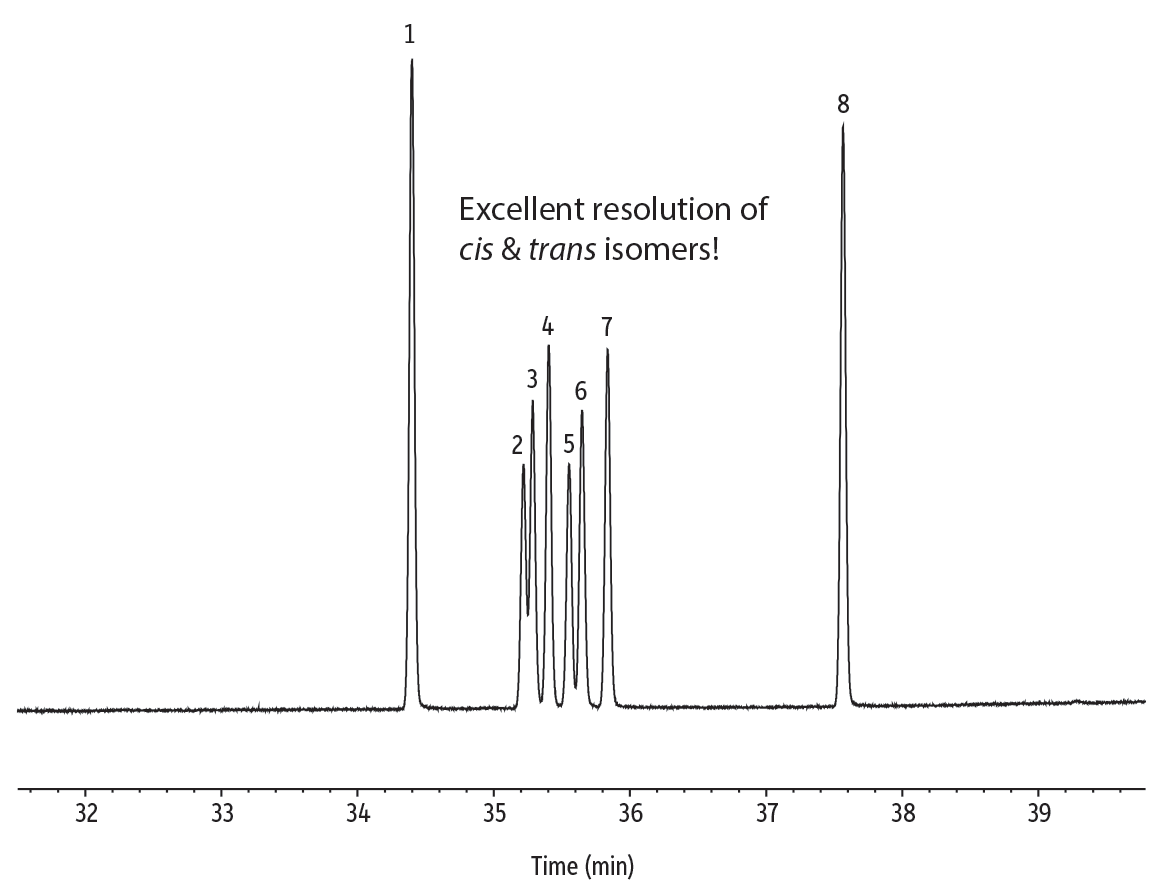
| Peaks | tR (min) | Conc. (mg/mL) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl stearate | 34.402 | 2.0 | C18:0 |
| 2. | Methyl petroselaidate | 35.220 | 0.8 | C18:1 (trans-6) |
| 3. | Methyl elaidate | 35.288 | 1.0 | C18:1 (trans-9) |
| 4. | Methyl trans-vaccenate | 35.405 | 1.2 | C18:1 (trans-11) |
| 5. | Methyl petroselinate | 35.555 | 0.8 | C18:1 (cis-6) |
| 6. | Methyl oleate | 35.650 | 1.0 | C18:1 (cis-9) |
| 7. | Methyl vaccenate | 35.838 | 1.2 | C18:1 (cis-11) |
| 8. | Methyl linoleate | 37.567 | 2.0 | C18:2 (cis-9,12) |
| Column | Rt-2560, 100 m, 0.25 mm ID, 0.20 µm (cat.# 13198) |
|---|---|
| Standard/Sample | cis/trans FAME mix (cat.# 35079) |
| Diluent: | Methylene chloride |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 200:1) |
| Liner: | Topaz 4 mm ID straight inlet liner w/ wool (cat.# 23300) |
| Inj. Temp.: | 240 °C |
| Oven | |
| Oven Temp.: | 100 °C (hold 4 min) to 240 °C at 3 °C/min (hold 10 min) |
| Carrier Gas | H2, constant flow |
| Flow Rate: | 2.2 mL/min |
| Detector | FID @ 250 °C |
|---|---|
| Instrument | Agilent 7890A GC |
Figure 6: Rt-2560 columns provide excellent separation of trans fats.
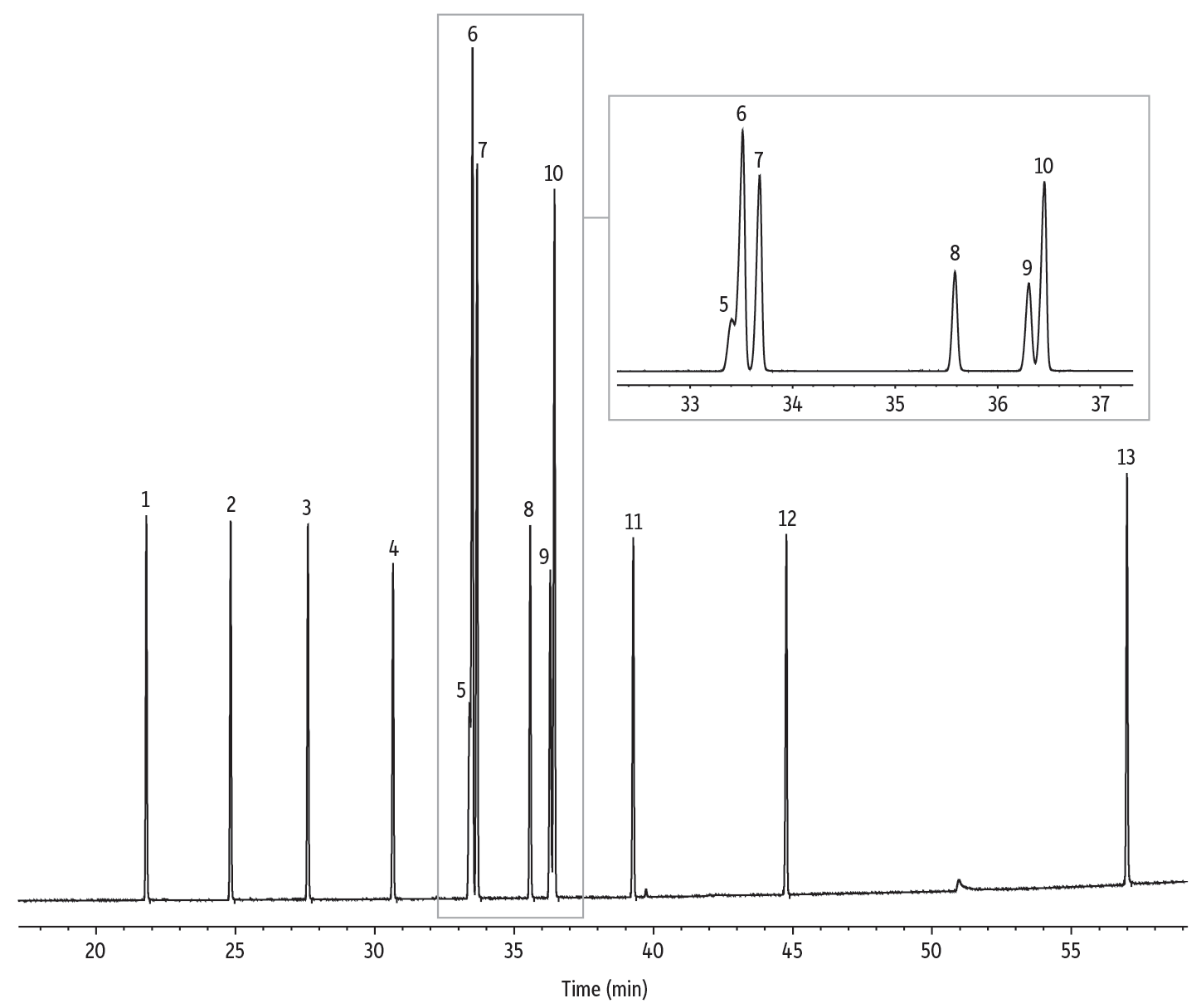
| Peaks | tR (min) | Conc. (wt.%) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl myristelaidate | 21.806 | 6 | C14:1 (trans-9) |
| 2. | Methyl trans-pentandecen-10-oate | 24.832 | 6 | C15:1 (trans-10) |
| 3. | Methyl palmitelaidate | 27.605 | 6 | C16:1 (trans-9) |
| 4. | Methyl trans-heptadecen-10-oate | 30.660 | 6 | C17:1 (trans-10) |
| 5. | Methyl petroselaidate | 33.407 | 4 | C18:1 (trans-6) |
| 6. | Methyl elaidate | 33.513 | 16 | C18:1 (trans-9) |
| Peaks | tR (min) | Conc. (wt.%) | Structural Nomenclature | |
|---|---|---|---|---|
| 7. | Methyl trans-vaccenate | 33.679 | 12 | C18:1 (trans-11) |
| 8. | Methyl linoelaidate | 35.583 | 6 | C18:2 (trans-9,12) |
| 9. | Methyl trans-nonadecen-7-oate | 36.303 | 6 | C19:1 (trans-7) |
| 10. | Methyl trans-nonadecen-10-oate | 36.455 | 12 | C19:1 (trans-10) |
| 11. | Methyl trans-eicosan-11-oate | 39.270 | 6 | C20:1 (trans-11) |
| 12. | Methyl brassidate | 44.764 | 6 | C21:1 (trans-13) |
| 13. | Methyl ricinelaidate | 56.987 | 8 | C18:1 (trans-9; OH-12) |
| Column | Rt-2560, 100 m, 0.25 mm ID, 0.20 µm (cat.# 13198) |
|---|---|
| Standard/Sample | |
| Diluent: | Hexane |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 200:1) |
| Liner: | Topaz 4.0 mm ID straight inlet liner w/wool (cat.# 23300) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 120 °C (hold 1 min) to 250 °C at 2 °C/min (hold 4 min) |
| Carrier Gas | H2, constant flow |
| Flow Rate: | 2 mL/min |
| Detector | FID @ 250 °C |
|---|---|
| Instrument | Agilent 7890A GC |
Figure 7: Animal fat-based FAMEs resolved, using an Rtx-2330 column.
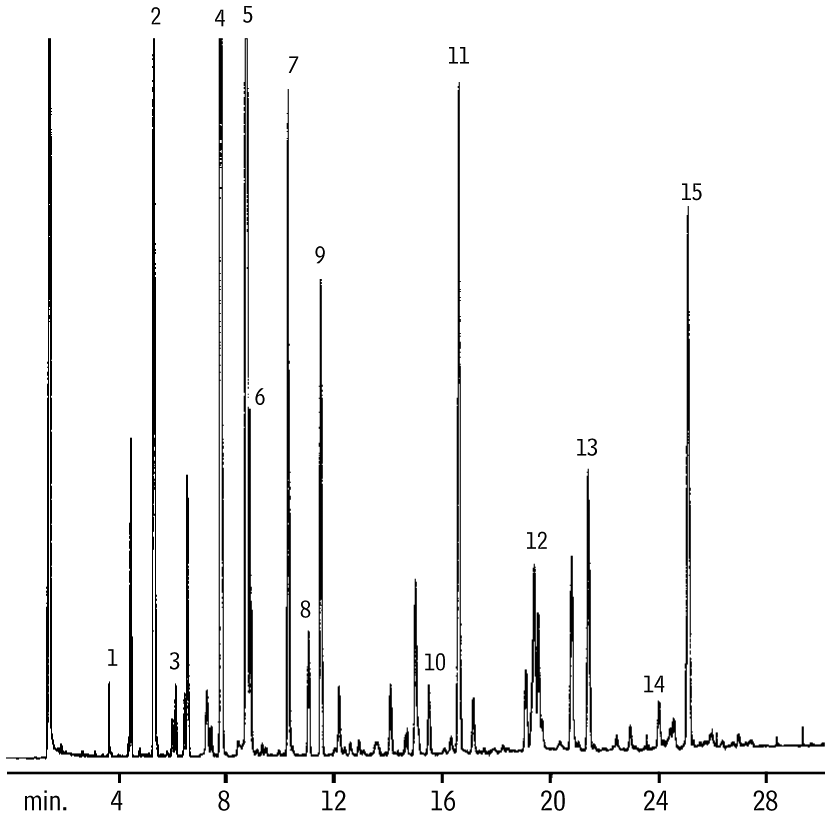
| Peaks | |
|---|---|
| 1. | C14:0 |
| 2. | C16:0 |
| 3. | C16:1n7 |
| 4. | C18:0 |
| 5. | C18:1n9 |
| 6. | C18:1n7 |
| 7. | C18:2n6 |
| Peaks | |
|---|---|
| 8. | C18:3n6 |
| 9. | C18:3n3 |
| 10. | C20:3n6 |
| 11. | C20:4n6 |
| 12. | C20:5n3 |
| 13. | C22:4n6 |
| 14. | C22:5n3 |
| 15. | C22:6n3 |
| Column | Rtx-2330, 30 m, 0.32 mm ID, 0.20 µm (cat.# 10724) |
|---|---|
| Standard/Sample | PUFA 2 mix |
| Injection | |
| Inj. Vol.: | 0.1 µL split (split ratio 20:1) |
| Inj. Temp.: | 260 °C |
| Oven | |
| Oven Temp.: | 160 °C to 250 °C at 2 °C/min (hold 10 min) |
| Carrier Gas | H2, constant pressure |
| Linear Velocity: | 40 cm/sec |
| Detector | FID @ 260 °C |
|---|---|
| Notes | FID sensitivity: 8 x 10-11 AFS |
Figure 8: Saw palmetto FAMEs resolved on an Rtx-WAX column.
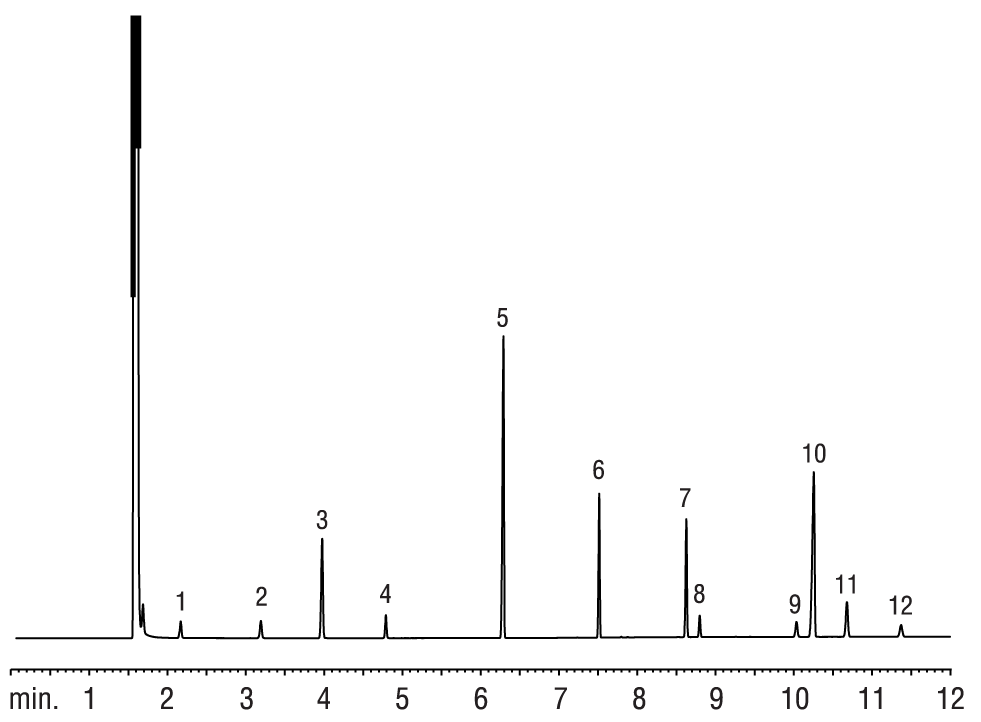
| Peaks | Conc. (mg/mL) | |
|---|---|---|
| 1. | Methyl caproate (C6:0) | 0.4 |
| 2. | Methyl caprylate (C8:0) | 0.4 |
| 3. | Methyl nonanoate (C9:0) | 2.0 |
| 4. | Methyl caprate (C10:0) | 0.4 |
| 5. | Methyl laurate (C12:0) | 5.0 |
| 6. | Methyl myristate (C14:0) | 2.0 |
| Peaks | Conc. (mg/mL) | |
|---|---|---|
| 7. | Methyl palmitate (C16:0) | 2.0 |
| 8. | Methyl palmitoleate (C16:1) | 0.4 |
| 9. | Methyl stearate (C18:0) | 0.4 |
| 10. | Methyl oleate (C18:1) | 5.0 |
| 11. | Methyl linoleate (C18:2) | 1.0 |
| 12. | Methyl linolenate (C18:3) | 0.4 |
| Column | Rtx-Wax, 30 m, 0.25 mm ID, 0.25 µm (cat.# 12423) |
|---|---|
| Standard/Sample | Saw palmetto standard |
| Conc.: | See peak list |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 100:1) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 120 °C (hold 3 min) to 220 °C at 20 °C/min (hold 12 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1 mL/min |
| Linear Velocity: | 34 cm/sec |
| Detector | FID @ 300 °C |
|---|
Figure 9: Olive oil FAMEs on a Stabilwax column.
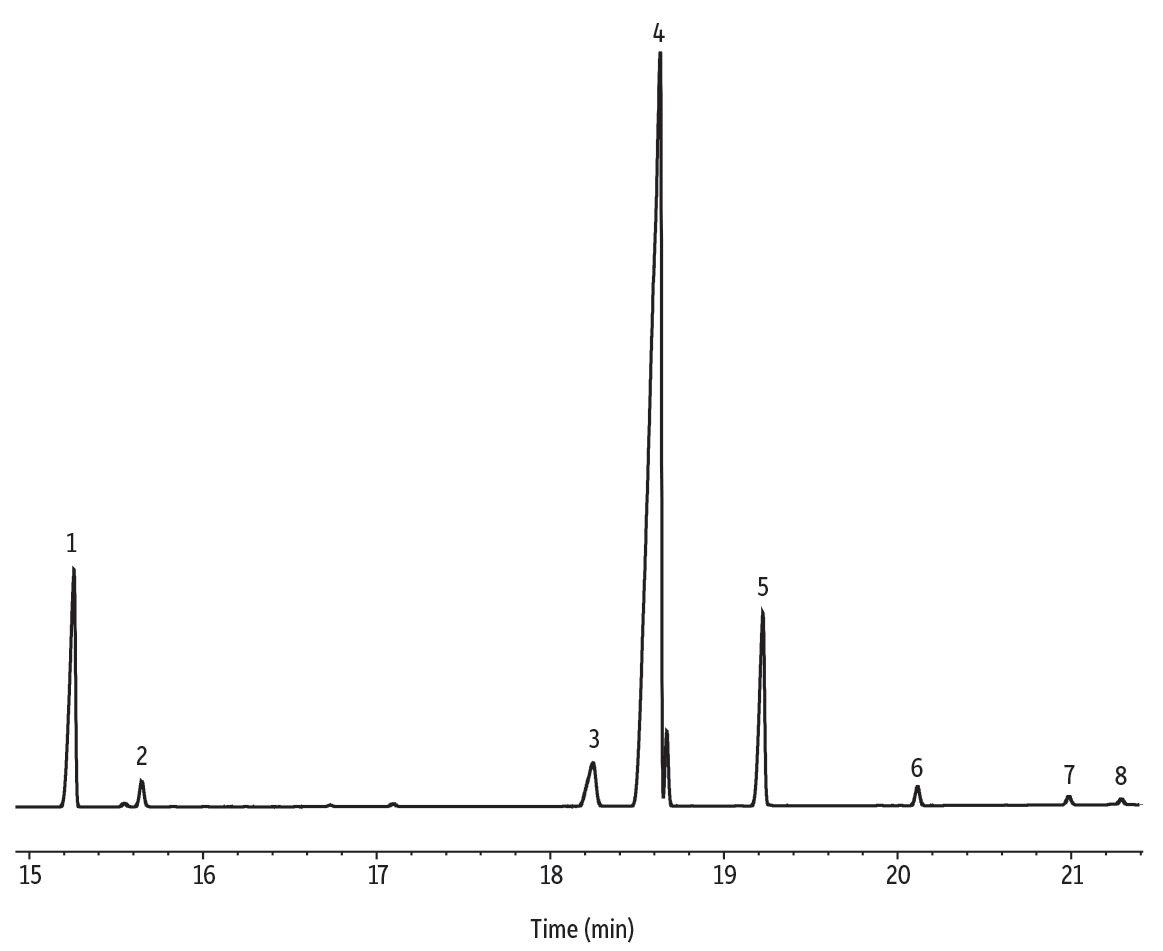
| Peaks | tR (min) | Conc. (wt.%) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl palmitate | 15.256 | 11.9 | C16:0 |
| 2. | Methyl palmitoleate | 15.646 | 1.0 | C16:1 (cis-9) |
| 3. | Methyl stearate | 18.244 | 3.2 | C18:0 |
| 4. | Methyl oleate | 18.631 | 73.2 | C18:1 (cis-9) |
| 5. | Methyl linoleate | 19.223 | 8.9 | C18:2 (cis-9,12) |
| 6. | Methyl linolenate | 20.114 | 0.8 | C18:3 (cis-9,12,15) |
| 7. | Methyl arachidate | 20.985 | 0.5 | C20:0 |
| 8. | Methyl eicosenoate | 21.287 | 0.4 | C20:1 (cis-11) |
| Column | Stabilwax, 60 m, 0.25 mm ID, 0.25 µm (cat.# 10626) |
|---|---|
| Standard/Sample | Olive oil reference standard (Sigma-Aldrich #47118) |
| Diluent: | Hexane |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 100:1) |
| Liner: | Topaz 4 mm ID straight inlet liner w/ wool (cat.# 23300) |
| Inj. Temp.: | 225 °C |
| Oven | |
| Oven Temp.: | 120 °C (hold 0.5 min) to 250 °C at 6 °C/min (hold 15 min) |
| Carrier Gas | H2, constant flow |
| Flow Rate: | 2 mL/min |
| Detector | FID @ 250 °C |
|---|---|
| Instrument | Agilent 7890A GC |
| Sample Preparation | The sample was prepared using methanolic HCl. |
Figure 10: Palm oil FAMEs on a Stabilwax column.
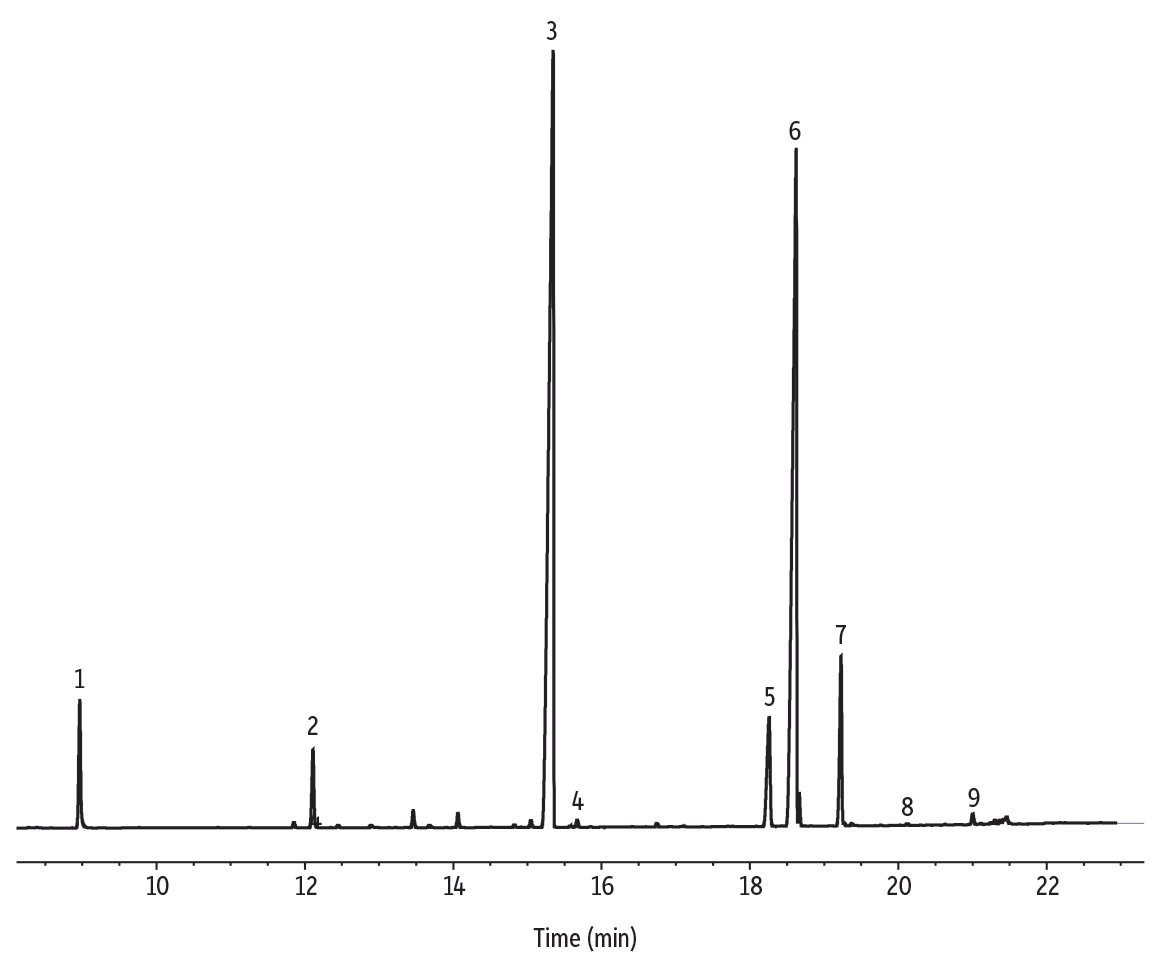
| Peaks | tR (min) | Conc. (wt.%) | Structural Nomenclature | |
|---|---|---|---|---|
| 1. | Methyl laurate | 8.963 | 3.0 | C12:0 |
| 2. | Methyl myristate | 12.106 | 2.0 | C14:0 |
| 3. | Methyl palmitate | 15.344 | 50.2 | C16:0 |
| 4. | Methyl palmitoleate | 15.668 | 1.5 | C16:1 (cis-9) |
| 5. | Methyl stearate | 18.254 | 4.4 | C18:0 |
| 6. | Methyl oleate | 18.620 | 34.1 | C18:1 (cis-9) |
| 7. | Methyl linoleate | 19.224 | 4.5 | C18:2 (cis9,12) |
| 8. | Methyl linolenate | 20.118 | 0.1 | C18:3 (cis-9,12,15) |
| 9. | Methyl arachidate | 21.001 | 0.2 | C20:0 |
| Column | Stabilwax, 60 m, 0.25 mm ID, 0.25 µm (cat.# 10626) |
|---|---|
| Standard/Sample | Palm oil standard (Supelco #46962) |
| Diluent: | Hexane |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 100:1) |
| Liner: | Topaz 4 mm ID straight inlet liner w/ wool (cat.# 23300) |
| Inj. Temp.: | 225 °C |
| Oven | |
| Oven Temp.: | 120 °C (hold 0.5 min) to 250 °C at 6 °C/min (hold 15 min) |
| Carrier Gas | H2, constant flow |
| Flow Rate: | 2 mL/min |
| Detector | FID @ 250 °C |
|---|---|
| Instrument | Agilent 7890A GC |
| Sample Preparation | The sample was prepared using methanolic HCl. |
Summary
Capillary GC is especially useful for determining total fat content, trans fat content, and total omega-3 polyunsaturated fatty acid content in foods. The choice of capillary column depends on the information required. For polyunsaturated FAMEs analysis, a FAMEWAX column allows fast, accurate quantification. A more polar Rt-2560 column is the column of choice when determining the total fat content, or the amount of trans fat, in an ingredient or end product.
Whatever your fatty acid analysis requirements, Restek can provide the consistent-performance analytical columns and reference mixes that will help you to accurately characterize your materials.
Composition of each mixture listed as a weight/weight % basis (minimum 50 mg/ampul)
| Compound Name |
Mix Cat. # | |||||||||
| FAME #1 35010 | FAME #2 35011 | FAME #3 35012 | FAME #4 35013 | FAME #5 35014 | FAME #6 35015 | FAME #7 35016 | FAME #8 35017 | FAME #9 35018 | FAME #12 35021 | |
| Methyl caproate (6:0) | 20.0 | 20.0 | ||||||||
| Methyl heptanoate (7:0) | 20.0 | |||||||||
| Methyl caprylate (8:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl nonanoate (9:0) | 20.0 | |||||||||
| Methyl caprate (10:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl undecanoate (11:0) | 20.0 | |||||||||
| Methyl laurate (12:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl tridecanoate (13:0) | 20.0 | 20.0 | ||||||||
| Methyl myristate (14:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl pentadecanoate (15:0) | 20.0 | 20.0 | ||||||||
| Methyl palmitate (16:0) | 20.0 | 20.0 | 20.0 | 20.0 | ||||||
| Methyl palmitoleate (16:1) | 20.0 | |||||||||
| Methyl heptadecanoate (17:0) | 20.0 | 20.0 | ||||||||
| Methyl stearate (18:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl oleate (18:1) | 20.0 | 20.0 | ||||||||
| Methyl linoleate (18:2) | 20.0 | |||||||||
| Methyl linolenate (18:3) | 20.0 | |||||||||
| Methyl nonadecanoate (19:0) | 20.0 | 20.0 | ||||||||
| Methyl arachidate (20:0) | 20.0 | 20.0 | 20.0 | |||||||
| Methyl eicosenoate (20:1) | 20.0 | 20.0 | ||||||||
| Methyl eicosadienoate (20:2) | 20.0 | |||||||||
| Methyl homo gamma linolenate (20:3) | 20.0 | |||||||||
| Methyl arachidonate (20:4) | 20.0 | |||||||||
| Methyl heneicosanoate (21:0) | 20.0 | |||||||||
| Methyl behenate (22:0) | 2.0 | |||||||||
| Methyl erucate (22:1) | 20.0 | |||||||||
| Methyl docosadienoate (22:2) | ||||||||||
| Methyl lignocerate (24:0) | 20.0 | |||||||||
| Methyl nervonate (24:1) | 20.0 | |||||||||
| Compound Name |
Mix Cat. # |
||||||||
| FAME #13 35034 | FAME #14 35035 | FAME #15 35036 | FAME #16 35022 | FAME #17 35023 | FAME #18 35024 | FAME #19 35025 | FAME #20 35026 | FAME #21 35027 | |
| Methyl caproate (6:0) | |||||||||
| Methyl heptanoate (7:0) | |||||||||
| Methyl caprylate (8:0) | 7.0 | ||||||||
| Methyl nonanoate (9:0) | |||||||||
| Methyl caprate (10:0) | 5.0 | ||||||||
| Methyl undecanoate (11:0) | |||||||||
| Methyl laurate (12:0) | 48.0 | ||||||||
| Methyl tridecanoate (13:0) | |||||||||
| Methyl myristate (14:0) | 0.1 | 1.0 | 15.0 | 2.0 | |||||
| Methyl pentadecanoate (15:0) | |||||||||
| Methyl palmitate (16:0) | 3.0 | 26.3 | 10.0 | 6.0 | 7.0 | 4.0 | 11.0 | 7.0 | 30.0 |
| Methyl palmitoleate (16:1) | 1.0 | 0.4 | 3.0 | ||||||
| Methyl heptadecanoate (17:0) | 0.3 | ||||||||
| Methyl stearate (18:0) | 2.0 | 33.7 | 3.0 | 3.0 | 5.0 | 3.0 | 3.0 | 3.0 | 14.0 |
| Methyl oleate (18:1) | 20.0 | 34.3 | 50.0 | 35.0 | 18.0 | 45.0 | 80.0 | 12.0 | 41.0 |
| Methyl linoleate (18:2) | 15.0 | 3.1 | 30.0 | 50.0 | 36.0 | 15.0 | 6.0 | 3.0 | 7.0 |
| Methyl linolenate (18:3) | 10.0 | 0.2 | 3.0 | 34.0 | 3.0 | 3.0 | |||
| Methyl nonadecanoate (19:0) | |||||||||
| Methyl arachidate (20:0) | 1.0 | 1.3 | 1.5 | 3.0 | 3.0 | ||||
| Methyl eicosenoate (20:1) | 10.0 | 0.1 | 1.5 | ||||||
| Methyl eicosadienoate (20:2) | 2.0 | ||||||||
| Methyl homo gamma linolenate (20:3) | |||||||||
| Methyl arachidonate (20:4) | |||||||||
| Methyl heneicosanoate (21:0) | |||||||||
| Methyl behenate (22:0) | 1.0 | 0.2 | 3.0 | 3.0 | |||||
| Methyl erucate (22:1) | 30.0 | 20.0 | |||||||
| Methyl docosadienoate (22:2) | 20.0 | ||||||||
| Methyl lignocerate (24:0) | 1.0 | 1.0 | 3.0 | ||||||
| Methyl nervonate (24:1) | 2.0 | ||||||||
References
- M.M. Mossoba, J.K.G. Kramer, P. Delmonte, M.P. Yurawecz, J.I. Rader, Official methods for the determination of trans fat, AOCS Press, 2003.
- AOCS Committee, Official methods and recommended practices of the AOCS, 7th edition, AOCS Press, 2017. https://www.aocs.org/store/shop-aocs/shop-aocs?productId=70978091.
- European pharmacopoeia (Ph. Eur.) 9th edition, European Pharmacopoeia, 2017. Book, online, PDF.
- K. Liu, Preparation of fatty acid methyl esters for gas‐chromatographic analysis of lipids in biological materials, J Am Oil Chem Soc 71 (11) (1994) 1179-1187. https://onlinelibrary.wiley.com/doi/abs/10.1007/BF02540534.
- K-D Müller, H.P. Nalik, E.N. Schmid, H. Husmann, G. Schomburg, Fast identification of mycobacterium species by GC analysis with trimethylsulfonium hydroxide (TMSH) for transesterification, J Sep Sci, 16 (3) (1993) 161-165. https://onlinelibrary.wiley.com/doi/abs/10.1002/jhrc.1240160306.
- K. Ichihara, Y. Fukubayashi, Preparation of fatty acid methyl esters for gas-liquid chromatography, J Lipid Res, 1 (3) (2010) 635–640. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2817593.
- K. iIchihara, C. Kohsaka, N. Tomari, T. Kiyono, J. Wada, K. Hirooka, Y. Yamamoto, Fatty acid analysis of triacylglycerols: Preparation of fatty acid methyl esters for gas chromatography Anal Biochem 495 (2016) 6-8. https://www.sciencedirect.com/science/article/pii/S0003269715005357

