Improved Screening Method for Acylcarnitines and Amino Acids in Dried Blood Spots by LC-MS/MS
Abstract
Newborn screening is critical in the clinical diagnosis of amino acid, fatty acid, and organic acid metabolism, allowing the diagnosis of over 30 metabolic diseases from a drop of blood sample. In this application note, we present a fast, single-step extraction LC-MS/MS screening approach with excellent chromatographic separation of 22 acylcarnitines and 13 amino acids in dried blood spots using a Raptor HILIC-Si 2.7 µm, 5 x 2.1 mm guard cartridge column. This method for acylcarnitines and amino acids provides improved sensitivity and specificity when compared to traditional flow injection-MS/MS methods with a fast total analysis time of just 1.2 minutes, without the need for sample derivatization.
Introduction
Newborn screening aims to detect congenital metabolic disorders like defects in amino acid metabolism, such as phenylketonuria (PKU) and maple syrup urine disease (MSUD), defects of fatty acid metabolism, and organic acidurias (isovaleric acidemia). If these metabolic disorders are not diagnosed early, they can lead to severe irreversible harm to newborns within their first few days of life. L-Carnitine (C0) assists coenzyme A (CoA)-activated medium- and long-chain fatty acids in mitochondrial transport. It forms fatty acylcarnitine esters by the action of carnitine acyltransferases that vary in chain length depending on their cellular location and metabolic purpose. These compounds play a crucial role in the production of energy for cell activities, and they serve as diagnostic markers for both fatty acid oxidation defects and organic acid disorders. Elevated acylcarnitine concentrations in human whole blood or plasma occur in response to a block in the metabolic pathway of either fatty acids or branched-chain amino acids. Therefore, acylcarnitine profile analysis is a useful clinical screening tool for the detection of several deficiencies in β-oxidation of fatty acids.
Newborn screening methods for acylcarnitines and amino acids typically include six to eleven amino acids and nine to twenty acylcarnitines. Dried blood spots (DBS) are the standard specimen used for testing due to easy sample collection, transportation, and storage. Currently, there are two widely used approaches in newborn screening methods for acylcarnitines and amino acids: with sample derivatization and without sample derivatization prior to flow injection-MS/MS analysis of solvent extracted DBS. However, these techniques suffer from partial hydrolysis of acylcarnitines during derivatization; time-consuming sample preparation; non-Gaussian peak shapes due to large injection volumes and very low flow rates; no retention; and poor separation and selectivity from matrix components, which causes decreased signal sensitivity for the target analytes. The combined result is that traditional screening approaches generate data that do not ensure certainty in diagnosis.
In order to address these drawbacks, and still provide the speed of flow injection approaches, we investigated a high-throughput assay with a simple sample preparation method (no derivatization) that allows the fast and reliable determination of 13 amino acids and 22 acylcarnitines from dried blood spot samples. This LC-MS/MS method for acylcarnitines and amino acids is unique in that it uses a short Raptor HILIC-Si guard cartridge column (2.7 µm, 5 x 2.1 mm) for chromatographic separations instead of a traditional scale analytical chromatography column. Compared to traditional flow injection analysis, in this approach results are generated in a fast total analysis time of 1.2 minutes with improved sensitivity and specificity with the help of chromatography. Sample preparation is based on effective extraction of the analytes from dried blood spot samples using organic solvent without the need for derivatization. This method can also separate the target analytes from matrix interference and cross talk from structurally similar compounds that are present in the method panel. The use of stable isotope-labeled internal standards ensures reliable and precise identification of the analytes.
Experimental
Human whole blood from four individuals was purchased from Bioreclamation. Whole blood from all the lots contained high endogenous levels of the majority of the acylcarnitines and amino acids; therefore, analyte standards were not fortified directly into the blood. All the analyte standards and internal standards were purchased from Cayman Chemical and Sigma-Aldrich. Dried blood spot samples were prepared by blotting the cards with 50 µL of human whole blood, the volume required to fill the pre-marked circle on the card for blood collection. DBS sampling simplifies sample collection, storage, and transfer, and it has the additional benefits of reduced contamination and a lower required blood volume. The samples were subjected to the following sample preparation method for acylcarnitines and amino acids.
DBS Sample Extraction Procedure
50 µL of human whole blood was blotted on to Whatman 903 neonatal protein saver cards to prepare the DBS samples. The cards were dried for one hour at room temperature, and a 3.0 mm disk (∼3.0 µL whole blood) was punched out using a DBS manual puncher into a 2.0 mL Eppendorf tube. 200 µL of an 85:15 acetonitrile:water solution containing known concentrations of 16 stable isotope-labeled internal standards was added to each tube. The sample was then vortexed and incubated for 20 minutes at room temperature on a microplate shaker. Following incubation, the samples were centrifuged for 10 minutes at 4000 rpm, and 150 µL of supernatant was filtered using Thomson SINGLE StEP Nano filter vials (cat.# 25882). The supernatant was then injected for LC-MS/MS analysis. This sample extraction procedure was suitable for all of the whole blood lots.
Optimization of DBS Sample Extraction Procedure
To achieve high recovery of all the analytes from the DBS sample as well as to ensure reduced matrix effects and good peak shapes for early eluting compounds, various sample extraction solvents were evaluated including 100% methanol, 90% methanol, 85% methanol, 80% methanol, 70% methanol, 60% methanol, 100% acetonitrile, 90% acetonitrile, 85% acetonitrile, 80% acetonitrile, and 70% acetonitrile prepared in water. 100% acetonitrile with 0.1 N hydrochloric acid was also evaluated. All the solvents were tested using the DBS sample extraction procedure described above and then injected for LC-MS/MS analysis, and the peak area responses and peak shapes were compared.
Among all the extraction solvents, the 85:15 acetonitrile:water (v/v) solvent was found to be the most effective overall for extraction of all the acylcarnitines and amino acids. Higher amino acids recovery was noticed with the extraction solvents containing more water due to their polarity, but this negatively impacted the extraction solubility of long-chain, nonpolar acylcarnitines. Therefore, 85:15 acetonitrile:water solvent is recommended for the coextraction method for acylcarnitines and amino acids from DBS. Another advantage of the 85:15 solvent mixture is that it is suitable for direct HILIC analysis without the need for evaporation and reconstitution to match conditions with the starting gradient of the mobile phase.
Instrument Conditions
This analytical method for acylcarnitines and amino acids was performed on a Shimadzu Nexera UHPLC equipped with a SCIEX API 4500 MS/MS. Mass spectrometric data were obtained in ESI positive ionization mode. Spray voltage was set to +3.5 kV, the capillary temperature was 600 °C, the curtain gas was 40.0, ion source gas 1 was 50.0, and ion source gas 2 was 40.0. Nitrogen collision gas was set to medium for all experiments. Instrument conditions and MRM transitions for all the analytes (acylcarnitines, amino acids, and internal standards) monitored are shown in the figures.
Results and Discussion
Chromatographic Performance
A fast, one-minute screening method for the analysis of 22 acylcarnitines (C2-C20) and 13 amino acids from dried blood spots along with 16 externally spiked deuterated internal standards was achieved using a Raptor HILIC-Si guard cartridge column. Use of the guard cartridge column in place of a traditional analytical column resulted in excellent retention and chromatographic separation in HILIC mode (Figure 1). The guard cartridge column is very easy to setup and can also be used as a prefilter to remove contaminants prior to injection.
The addition of ammonium formate to the aqueous mobile phase improved peak shape and peak response for most analytes. Detection was performed using positive ESI mode, due to the large number of analytes (51 compounds). Scheduled MRM was chosen, and scan rates and retention time windows were optimized to ensure that more than seven data points were collected per peak; this allowed for improved sensitivity and greater confidence in the resulting data. In contrast with flow injection-MS/MS analysis, the Raptor HILIC-Si guard cartridge column not only provided stable analyte retention and fast chromatographic separation for all 35 analytes and 16 internal standards but also mitigated matrix interferences from whole blood.
Figure 1: Optimized screening method for 22 acylcarnitines and 13 amino acids at endogenous levels in dried blood spot samples with 16 deuterated internal standards using a Raptor HILIC-Si guard cartridge column.
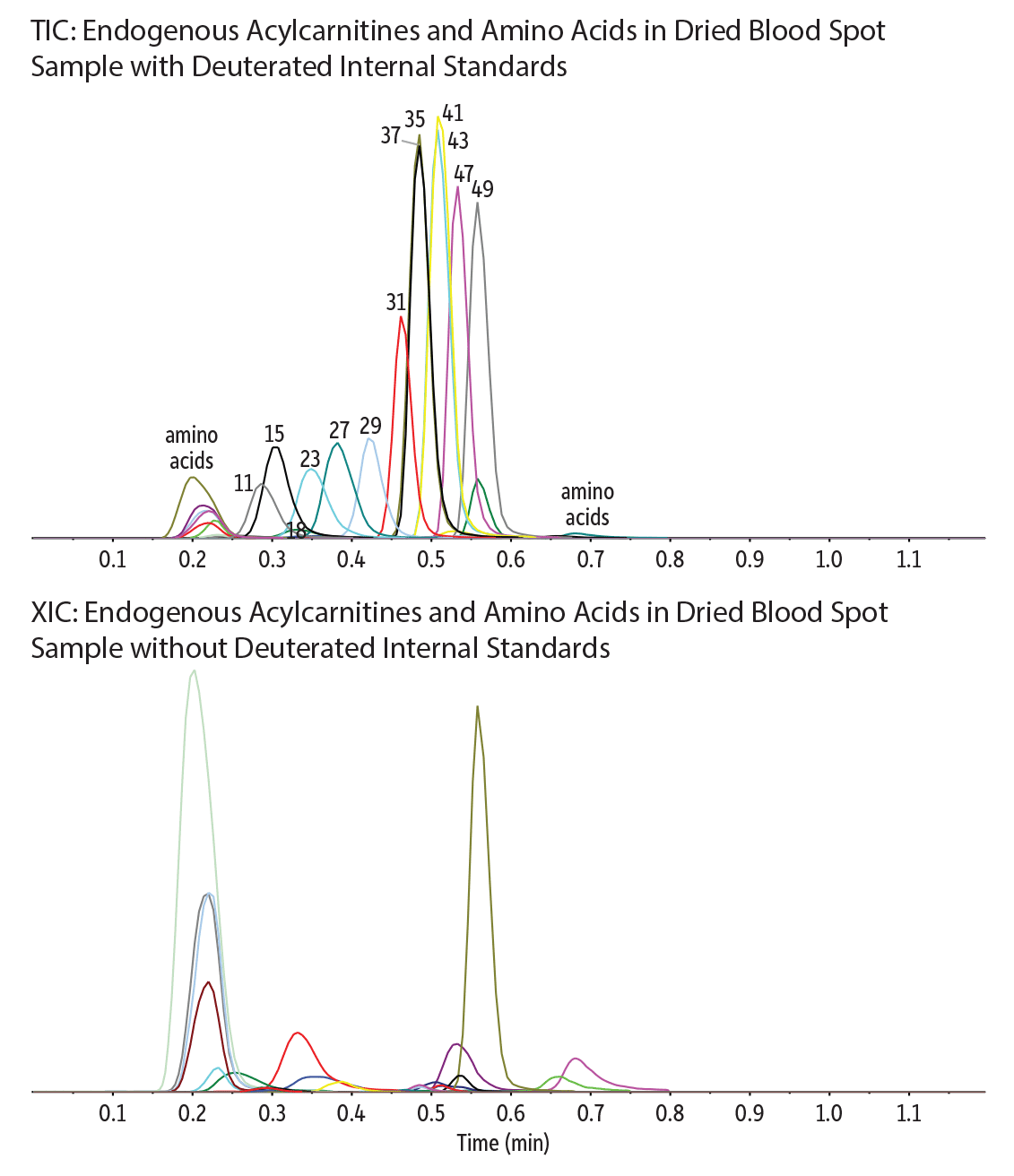
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Phenylalanine | 0.20 | 166.0 | 120.1 |
| 2. | Leucine | 0.21 | 132.1 | 86.0 |
| 3. | Leucine-d3 | 0.21 | 135.2 | 89.1 |
| 4. | Isoleucine | 0.21 | 132.1 | 86.1 |
| 5. | Tyrosine | 0.22 | 182.1 | 91.0 |
| 6. | Methionine | 0.24 | 150.1 | 56.1 |
| 7. | Methionine-d3 | 0.24 | 153.2 | 107.1 |
| 8. | Valine | 0.27 | 118.1 | 72.0 |
| 9. | C20-Eicosanoyl-L-carnitine | 0.27 | 456.4 | 85.1 |
| 10. | C18-Stearoyl-L-carnitine | 0.28 | 428.3 | 85.1 |
| 11. | C18-Stearoyl-L-carnitine-d3 | 0.29 | 431.4 | 85.1 |
| 12. | C18:1 Oleoyl-L-carnitine | 0.29 | 426.4 | 85.1 |
| 13. | C18:2 Linoleoyl-L-carnitine | 0.30 | 424.3 | 85.1 |
| 14. | C16-Palmitoyl-L-carnitine | 0.30 | 400.3 | 85.1 |
| 15. | C16-Palmitoyl-L-carnitine-d3 | 0.31 | 403.3 | 85.1 |
| 16. | C16:1 Palmitolelyl-L-carnitine | 0.31 | 398.3 | 85.1 |
| 17. | C14-Myristoyl-L-carnitine | 0.32 | 372.3 | 85.1 |
| 18. | C14-Myristoyl-L-carnitine-d3 | 0.33 | 375.3 | 85.1 |
| 19. | C14:1 Tetradecenoyl-L-carnitine | 0.33 | 370.3 | 85.1 |
| 20. | C14:2-Tetradecadienoyl-L-carnitine | 0.33 | 368.3 | 85.1 |
| 21. | Proline | 0.33 | 116.0 | 70.1 |
| 22. | C12-Lauroyl-L-carnitine | 0.35 | 344.3 | 85.1 |
| 23. | C12-Lauroyl-L-carnitine-d3 | 0.35 | 347.3 | 85.1 |
| 24. | Alanine | 0.36 | 90.1 | 44.1 |
| 25. | Alanine-d4 | 0.35 | 94.1 | 48.1 |
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 26. | C10-Decanoyl-L-carnitine | 0.38 | 316.3 | 85.1 |
| 27. | C10-Decanoyl-L-carnitine-d3 | 0.39 | 319.2 | 85.1 |
| 28. | C8-Octanoyl-L-carnitine | 0.43 | 288.3 | 85.1 |
| 29. | C8-Octanoyl-L-carnitine-d3 | 0.43 | 291.2 | 85.1 |
| 30. | C7-Heptanoyl-L-carnitine | 0.45 | 274.2 | 85.1 |
| 31. | C6-Hexanoyl-L-carnitine-d3 | 0.46 | 263.2 | 85.1 |
| 32. | C6-Hexanoyl-L-carnitine | 0.47 | 260.2 | 85.1 |
| 33. | Glutamine | 0.45 | 147.1 | 84.1 |
| 34. | C5-Valeryl-L-carnitine | 0.48 | 246.2 | 85.1 |
| 35. | C5-Valeryl-L-carnitine-d3 | 0.49 | 249.1 | 85.1 |
| 36. | C5-Isovaleryl-L-carnitine | 0.49 | 246.1 | 85.1 |
| 37. | C5-Isovaleryl-L-carnitine-d3 | 0.49 | 249.2 | 85.1 |
| 38. | 2-Methylbutyryl-L-carnitine | 0.49 | 246.2 | 85.1 |
| 39. | C5:1-Tiglyl-L-carnitine | 0.50 | 244.2 | 85.1 |
| 40. | C4-Butyryl-L-carnitine | 0.51 | 232.2 | 85.1 |
| 41. | C4-Butyryl-L-carnitine-d3 | 0.51 | 235.2 | 85.1 |
| 42. | C4-Isobutyryl-L-carnitine | 0.51 | 232.1 | 85.1 |
| 43. | C4-Isobutyryl-L-carnitine-d3 | 0.51 | 235.1 | 85.1 |
| 44. | Citrulline | 0.51 | 176.1 | 113.1 |
| 45. | Glutamic acid | 0.55 | 148.1 | 83.9 |
| 46. | C3-Propionyl-L-carnitine | 0.54 | 218.1 | 85.1 |
| 47. | C3-Propionyl-L-carnitine-d3 | 0.54 | 221.2 | 85.1 |
| 48. | C2-Acetyl-L-carnitine | 0.56 | 204.1 | 85.1 |
| 49. | C2-Acetyl-L-carnitine-d3 | 0.56 | 207.1 | 85.1 |
| 50. | Arginine | 0.66 | 175.2 | 70.1 |
| 51. | Ornithine | 0.69 | 133.1 | 70.1 |
| Column | Raptor HILIC-Si EXP guard cartridge column (cat.# 9310A0252) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 5 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 85:15 Acetonitrile:water (v/v) | ||||||||||||||||||||
| Conc.: | Endogenous levels | ||||||||||||||||||||
| Inj. Vol.: | 2.0 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 30 mM Ammonium formate in water | ||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | UHPLC |
| Sample Preparation | 50 µL of whole blood was spotted on to Whatman 903 neonatal protein saver cards, which were then dried for 1 hour at room temperature. A 3.0 mm disk (∼3.0 µL whole blood) was punched out of the dried spot and into a 2.0 mL Eppendorf tube. 200 µL of 85:15 acetonitrile:water (v/v) that was fortified with known concentrations of stable isotope-labeled internal standards was added, and then the sample was vortexed and incubated for 20 minutes at room temperature on a microplate shaker at a speed of 400 rpm. The sample was then centrifuged for 10 minutes at 4000 rpm, and 150 µL of the supernatant was filtered using a Thomson SINGLE StEP Nano filter vial (cat.# 25882) prior to LC-MS/MS analysis. |
Flow Injection-MS/MS vs. LC-MS/MS
To demonstrate the magnitude of matrix interference and the improvement in data quality obtained using LC-MS/MS with a guard cartridge column, DBS samples were extracted and analyzed first by flow injection-MS/MS analysis (Figure 2) using 100% methanol with 0.1% formic acid (v/v) at a 0.3 mL/min flow rate, which is a commonly used method in labs. Then, the rapid LC-MS/MS analysis utilizing the Raptor HILIC-Si guard cartridge column at 0.5 mL/min (Figure 1) was run using the same sample, and results were compared. The rapid LC-MS/MS method showed reproducible retention and selectivity and also increased signal-to-noise ratios and signal intensities for acylcarnitines by two- to fivefold, and for amino acids by tenfold when compared to flow injection analysis. The flow injection analysis (Figure 2) showed elevated baselines instead of a peak for a few amino acids and broad, non-Gaussian peaks that could negatively impact peak integration and identification, thereby producing potentially inconclusive data. This comparison illustrates that the fast LC-MS/MS technique will allow both primary and secondary tier testing in a single run without the need for sample derivatization.
Figure 2: Typical flow injection-MS/MS method for acylcarnitines and amino acids in dried blood spots.
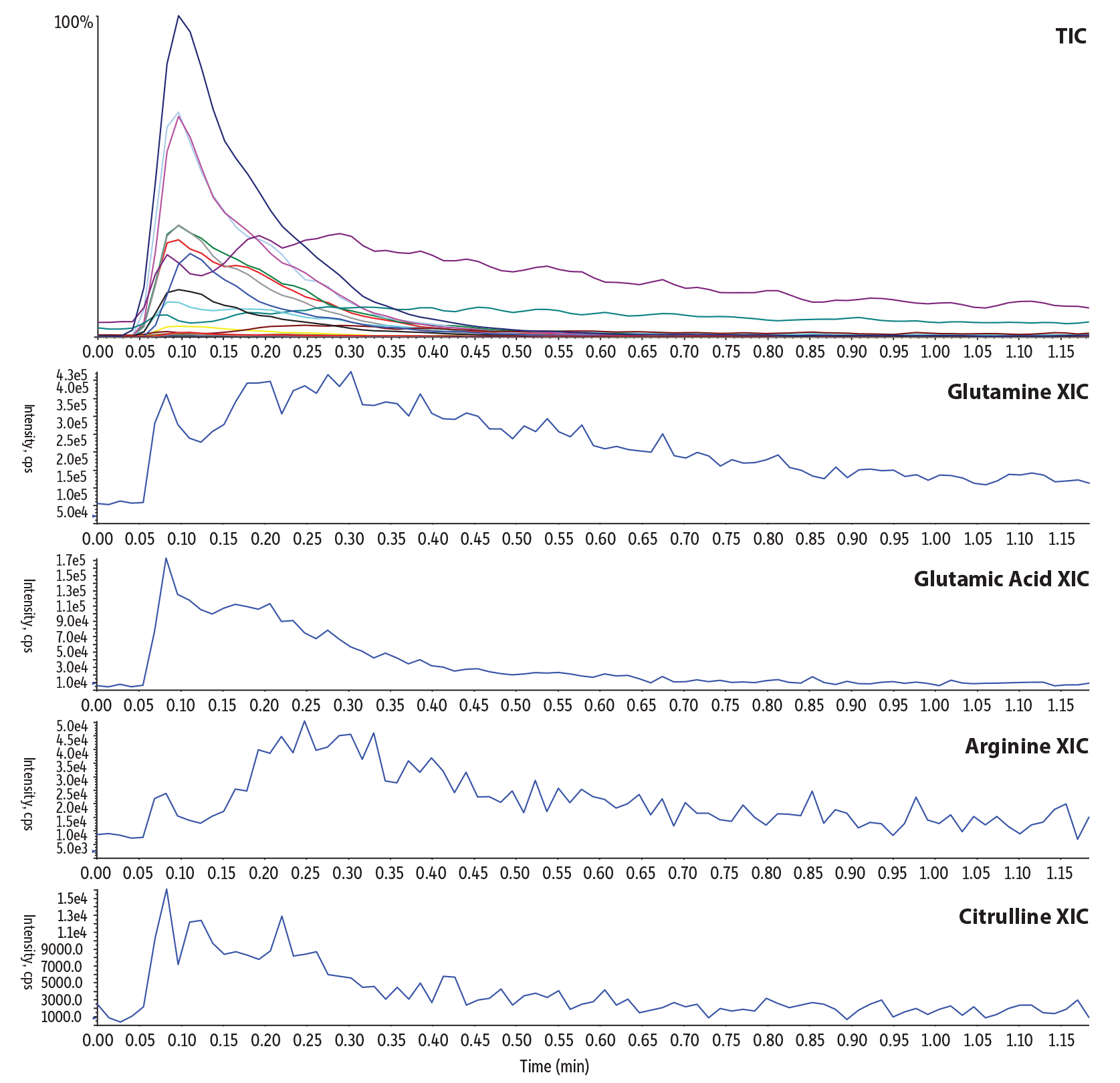
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Phenylalanine | 0.1 | 166.0 | 120.1 |
| 2. | Leucine | 0.1 | 132.1 | 86.0 |
| 3. | Isoleucine | 0.1 | 132.1 | 86.1 |
| 4. | Tyrosine | 0.1 | 182.1 | 91.0 |
| 5. | Methionine | 0.1 | 150.1 | 56.1 |
| 6. | Valine | 0.1 | 118.1 | 72.0 |
| 7. | C20-Eicosanoyl-L-carnitine | 0.1 | 456.4 | 85.1 |
| 8. | C18-Stearoyl-L-carnitine | 0.1 | 428.3 | 85.1 |
| 9. | C18:1 Oleoyl-L-carnitine | 0.1 | 426.4 | 85.1 |
| 10. | C18:2 Linoleoyl-L-carnitine | 0.1 | 424.3 | 85.1 |
| 11. | C16-Palmitoyl-L-carnitine | 0.1 | 400.3 | 85.1 |
| 12. | C16:1 Palmitolelyl-L-carnitine | 0.1 | 398.3 | 85.1 |
| 13. | C14-Myristoyl-L-carnitine | 0.1 | 372.3 | 85.1 |
| 14. | C14:1 Tetradecenoyl-L-carnitine | 0.1 | 370.3 | 85.1 |
| 15. | C14:2-Tetradecadienoyl-L-carnitine | 0.1 | 368.3 | 85.1 |
| 16. | Proline | 0.1 | 116.0 | 70.1 |
| 17. | C12-Lauroyl-L-carnitine | 0.1 | 344.3 | 85.1 |
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 18. | Alanine | 0.1 | 90.1 | 44.1 |
| 19. | C10-Decanoyl-L-carnitine | 0.1 | 316.3 | 85.1 |
| 20. | C8-Octanoyl-L-carnitine | 0.1 | 288.3 | 85.1 |
| 21. | C7-Heptanoyl-L-carnitine | 0.1 | 274.2 | 85.1 |
| 22. | C6-Hexanoyl-L-carnitine | 0.1 | 260.2 | 85.1 |
| 23. | Glutamine | 0.1 | 147.1 | 84.1 |
| 24. | C5-Valeryl-L-carnitine | 0.1 | 246.2 | 85.1 |
| 25. | C5-Isovaleryl-L-carnitine | 0.1 | 246.1 | 85.1 |
| 26. | 2-Methylbutyryl-L-carnitine | 0.1 | 246.2 | 85.1 |
| 27. | C5:1-Tiglyl-L-carnitine | 0.1 | 244.2 | 85.1 |
| 28. | C4-Butyryl-L-carnitine | 0.1 | 232.2 | 85.1 |
| 29. | C4-Isobutyryl-L-carnitine | 0.1 | 232.1 | 85.1 |
| 30. | Citrulline | 0.1 | 176.1 | 113.1 |
| 31. | Glutamic acid | 0.1 | 148.1 | 83.9 |
| 32. | C3-Propionyl-L-carnitine | 0.1 | 218.1 | 85.1 |
| 33. | C2-Acetyl-L-carnitine | 0.1 | 204.1 | 85.1 |
| 34. | Arginine | 0.1 | 175.2 | 70.1 |
| 35. | Ornithine | 0.1 | 133.1 | 70.1 |
| Column | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 30 °C | ||||||||||||
| Standard/Sample | |||||||||||||
| Diluent: | 85:15 Acetonitrile:water (v/v) | ||||||||||||
| Inj. Vol.: | 2.0 µL | ||||||||||||
| Mobile Phase | |||||||||||||
| B: | 0.1% Formic acid in methanol | ||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | UHPLC |
| Sample Preparation | 50 µL of whole blood was spotted on to Whatman 903 neonatal protein saver cards, which were then dried for 1 hour at room temperature. A 3.0 mm disk (∼3.0 µL whole blood) was punched out of the dried spot and into a 2.0 mL Eppendorf tube. 200 µL of 85:15 acetonitrile:water (v/v) that was fortified with known concentrations of stable isotope-labeled internal standards was added, and then the sample was vortexed and incubated for 20 minutes at room temperature on a microplate shaker at a speed of 400 rpm. The sample was then centrifuged for 10 minutes at 4000 rpm, and 150 µL of the supernatant was filtered using a Thomson SINGLE StEP Nano filter vial (cat.# 25882) prior to flow injection-MS/MS analysis. |
Addressing Specificity and Cross Talk: the Importance of Chromatography
Structurally similar compounds like glutamine and glutamic acid can form isobaric product ions, resulting in a cross-talk peak in the glutamic acid transition at 0.55 min from glutamine, which elutes at 0.45 minutes (Figure 3A). Additionally, a high-intensity isobaric matrix interference was observed in whole blood for the glutamine transition at 0.66 minutes (Figure 3B). No interference signal was evident in the solvent standard (Figure 3A), confirming that the interference is from the matrix. Due to the low endogenous levels of glutamine in whole blood, we fortified whole blood with 500 ppb glutamine (Figure 3C) to prove that the Raptor HILIC-Si guard cartridge column can separate the isobaric matrix peak from glutamine to avoid potentially false positive results. With the help of scheduled MRM, we have the advantage of selectively identifying glutamine only even at low levels based on its retention time and can easily prevent any negative impact from the strong matrix peak on the analyte peak area or signal (Figure 3D). In contrast, with the flow injection-MS/MS analysis, the same sample would have given a possible false positive result for glutamine due to the high matrix interference signal in the glutamine transition because there is no chromatographic separation (Figure 2).
Figure 3: Specificity and separation from matrix interference of structural isomer amino acids. (XIC overlay of glutamine and glutamic acid MRM channels.)
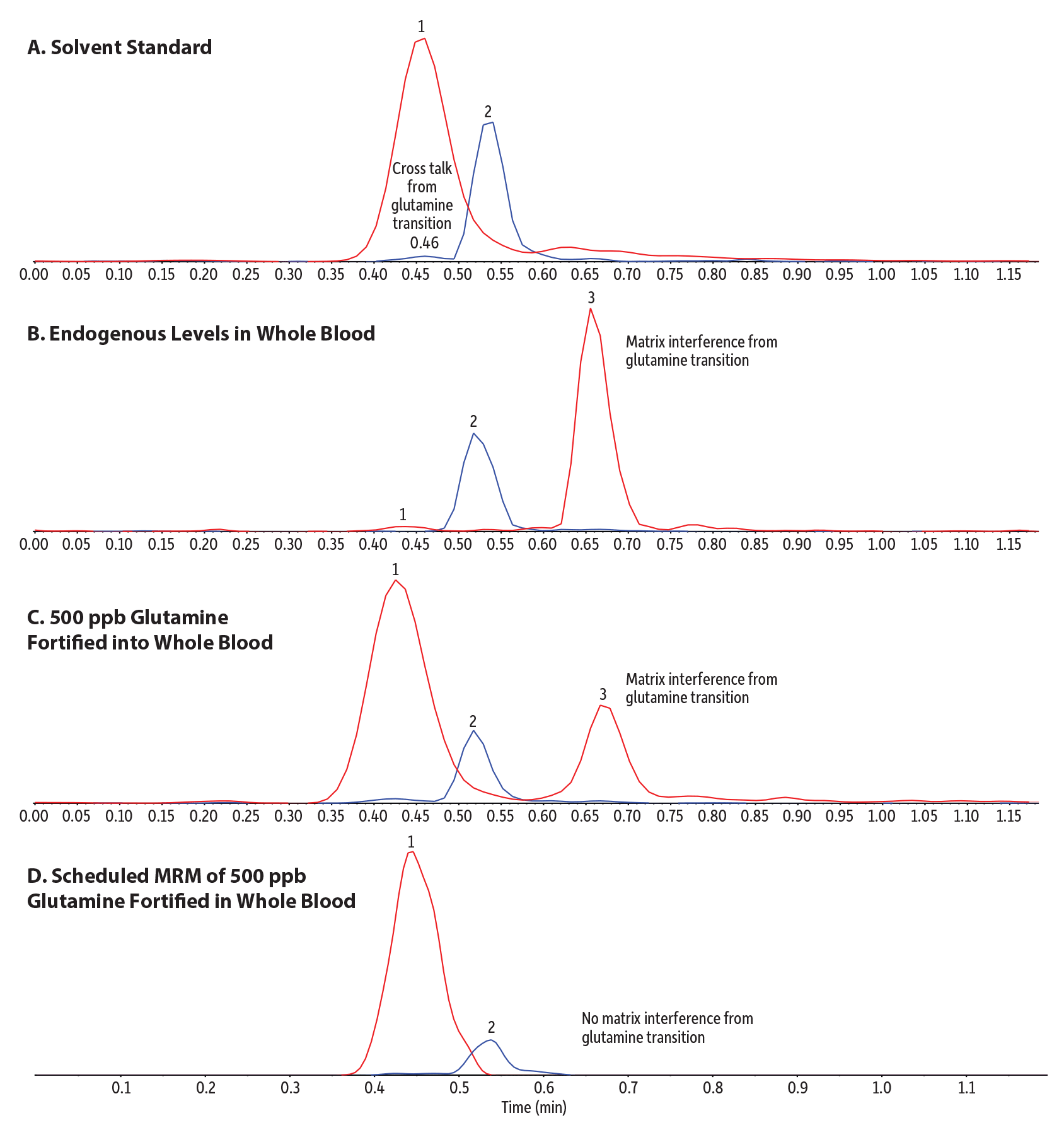
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Glutamine | 0.45 | 147.1 | 84.1 |
| 2. | Glutamic acid | 0.55 | 148.1 | 83.9 |
| 3. | Matrix interference | 0.66 | 147.1 | 84.1 |
| Column | Raptor HILIC-Si EXP guard cartridge column (cat.# 9310A0252) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 5 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 85:15 Acetonitrile:water (v/v) | ||||||||||||||||||||
| Inj. Vol.: | 2.0 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 30 mM Ammonium formate in water | ||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | UHPLC |
| Sample Preparation | 50 µL of whole blood was spotted on to Whatman 903 neonatal protein saver cards, which were then dried for 1 hour at room temperature. A 3.0 mm disk (∼3.0 µL whole blood) was punched out of the dried spot and into a 2.0 mL Eppendorf tube. 200 µL of 85:15 acetonitrile:water (v/v) that was fortified with known concentrations of stable isotope-labeled internal standards was added, and then the sample was vortexed and incubated for 20 minutes at room temperature on a microplate shaker at a speed of 400 rpm. The sample was then centrifuged for 10 minutes at 4000 rpm, and 150 µL of the supernatant was filtered using a Thomson SINGLE StEP Nano filter vial (cat.# 25882) prior to LC-MS/MS analysis. |
| Notes | Figure Descriptions A. LC-MS/MS analysis of a 100 ng/mL glutamine and glutamic acid standard prepared in 85:15 acetonitrile:water using a Raptor HILIC-Si guard cartridge column without scheduled MRM. B. LC-MS/MS analysis of a blank dried blood spot sample containing endogenous levels of glutamine and glutamic acid using a Raptor HILIC-Si guard cartridge column without scheduled MRM. C. LC-MS/MS analysis of a blank dried blood spot sample fortified with 500 ppb glutamine only (glutamine and glutamic acid are present endogenously) using a Raptor HILIC-Si guard cartridge column without scheduled MRM. D. LC-MS/MS analysis of a blank dried blood spot sample fortified with 500 ppb glutamine only (glutamine and glutamic acid present endogenously) using a Raptor HILIC-Si guard cartridge column with scheduled MRM. |
Method Robustness
Following 450 injections of a dried blood spot sample prepared from whole blood, the chromatographic peaks of all the analytes and internal standards maintained their initial peak shape, retention time, and intensity (Figure 4). A maximum back pressure of 1800 psi was reached after 10 matrix equilibration injections, and it did not change significantly over the course of the injection series, indicating no issues with clogging of the column had occurred. Sample filtration was an important factor in preventing blockages because no other cleanup steps were performed. The high degree of stability and repeatability demonstrated here make the proposed method conditions for acylcarnitines and amino acids amenable to high-throughput screening.
Figure 4: Robust column performance over 450 injections of dried blood spot sample extracts.
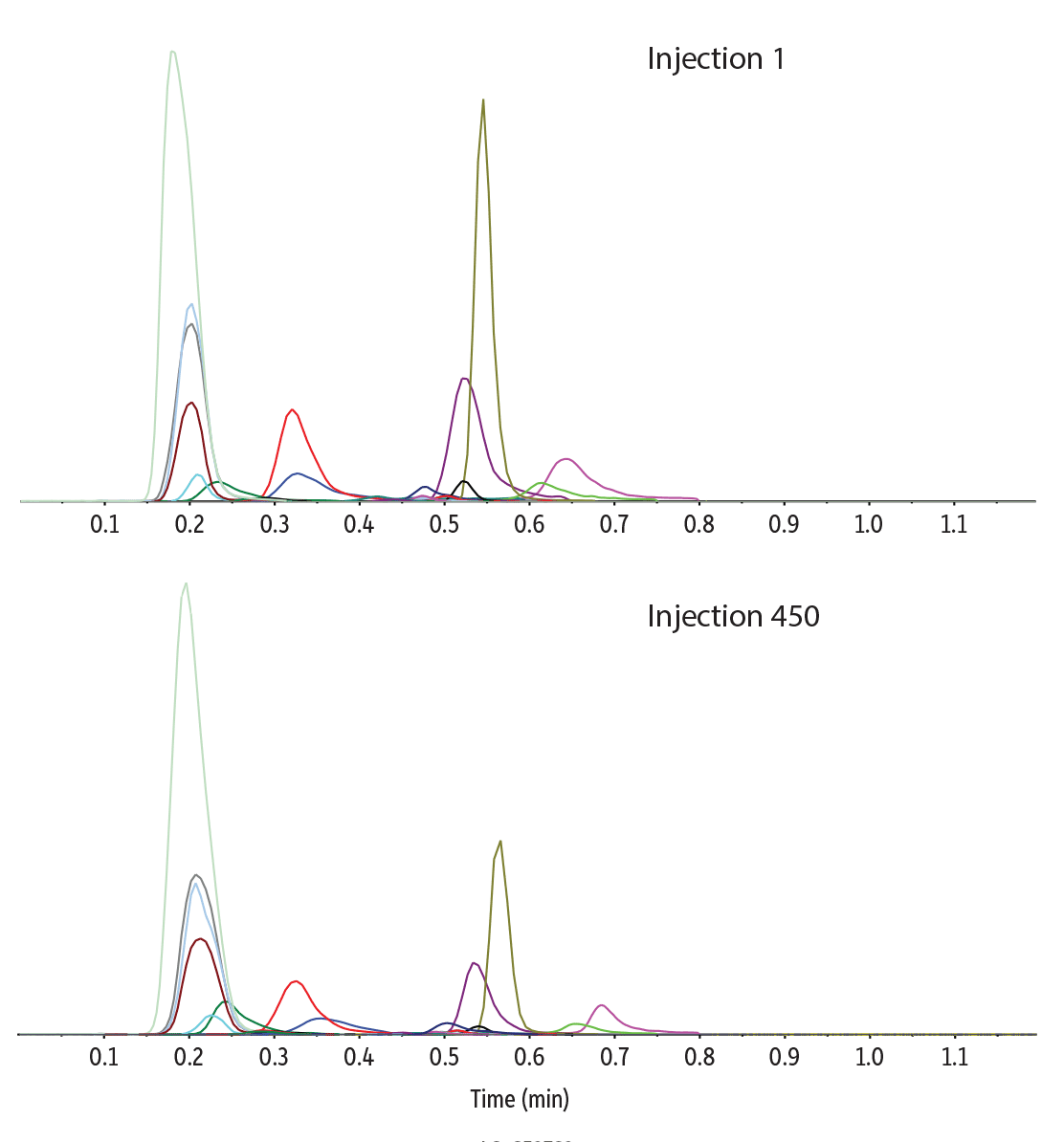
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Phenylalanine | 0.20 | 166.0 | 120.1 |
| 2. | Leucine | 0.21 | 132.1 | 86.0 |
| 3. | Isoleucine | 0.21 | 132.1 | 86.1 |
| 4. | Tyrosine | 0.22 | 182.1 | 91.0 |
| 5. | Methionine | 0.24 | 150.1 | 56.1 |
| 6. | Valine | 0.27 | 118.1 | 72.0 |
| 7. | C20-Eicosanoyl-L-carnitine | 0.27 | 456.4 | 85.1 |
| 8. | C18-Stearoyl-L-carnitine | 0.28 | 428.3 | 85.1 |
| 9. | C18:1 Oleoyl-L-carnitine | 0.29 | 426.4 | 85.1 |
| 10. | C18:2 Linoleoyl-L-carnitine | 0.30 | 424.3 | 85.1 |
| 11. | C16-Palmitoyl-L-carnitine | 0.30 | 400.3 | 85.1 |
| 12. | C16:1 Palmitolelyl-L-carnitine | 0.31 | 398.3 | 85.1 |
| 13. | C14-Myristoyl-L-carnitine | 0.32 | 372.3 | 85.1 |
| 14. | C14:1 Tetradecenoyl-L-carnitine | 0.33 | 370.3 | 85.1 |
| 15. | C14:2-Tetradecadienoyl-L-carnitine | 0.33 | 368.3 | 85.1 |
| 16. | Proline | 0.33 | 116.0 | 70.1 |
| 17. | C12-Lauroyl-L-carnitine | 0.35 | 344.3 | 85.1 |
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 18. | Alanine | 0.36 | 90.1 | 44.1 |
| 19. | C10-Decanoyl-L-carnitine | 0.38 | 316.3 | 85.1 |
| 20. | C8-Octanoyl-L-carnitine | 0.43 | 288.3 | 85.1 |
| 21. | C7-Heptanoyl-L-carnitine | 0.45 | 274.2 | 85.1 |
| 22. | C6-Hexanoyl-L-carnitine | 0.47 | 260.2 | 85.1 |
| 23. | Glutamine | 0.45 | 147.1 | 84.1 |
| 24. | C5-Valeryl-L-carnitine | 0.48 | 246.2 | 85.1 |
| 25. | C5-Isovaleryl-L-carnitine | 0.49 | 246.1 | 85.1 |
| 26. | 2-Methylbutyryl-L-carnitine | 0.49 | 246.2 | 85.1 |
| 27. | C5:1-Tiglyl-L-carnitine | 0.50 | 244.2 | 85.1 |
| 28. | C4-Butyryl-L-carnitine | 0.51 | 232.2 | 85.1 |
| 29. | C4-Isobutyryl-L-carnitine | 0.51 | 232.1 | 85.1 |
| 30. | Citrulline | 0.51 | 176.1 | 113.1 |
| 31. | Glutamic acid | 0.55 | 148.1 | 83.9 |
| 32. | C3-Propionyl-L-carnitine | 0.54 | 218.1 | 85.1 |
| 33. | C2-Acetyl-L-carnitine | 0.56 | 204.1 | 85.1 |
| 34. | Arginine | 0.66 | 175.2 | 70.1 |
| 35. | Ornithine | 0.69 | 133.1 | 70.1 |
| Column | Raptor HILIC-Si EXP guard cartridge column (cat.# 9310A0252) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 5 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 85:15 Acetonitrile:water (v/v) | ||||||||||||||||||||
| Inj. Vol.: | 2.0 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 30 mM Ammonium formate in water | ||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | UHPLC |
| Sample Preparation | 50 µL of whole blood was spotted on to Whatman 903 neonatal protein saver cards, which were then dried for 1 hour at room temperature. A 3.0 mm disk (∼3.0 µL whole blood) was punched out of the dried spot and into a 2.0 mL Eppendorf tube. 200 µL of 85:15 acetonitrile:water (v/v) that was fortified with known concentrations of stable isotope-labeled internal standards was added, and then the sample was vortexed and incubated for 20 minutes at room temperature on a microplate shaker at a speed of 400 rpm. The sample was then centrifuged for 10 minutes at 4000 rpm, and 150 µL of the supernatant was filtered using a Thomson SINGLE StEP Nano filter vial (cat.# 25882) prior to LC-MS/MS analysis. |
Conclusion
In summary, conditions for a fast 1.2-minute LC-MS/MS neonatal screening method for acylcarnitines and amino acids in dried blood spots were evaluated using a Raptor HILIC-Si guard cartridge column. Sample preparation was optimized to obtain the highest recovery of endogenously present analytes of both types in whole blood. This method has been demonstrated to be simple, fast, robust, and easy to perform. While it is comparable in speed to flow injection-MS/MS analysis, it offers several important advantages, including a derivatization-free sample preparation and a substantial improvement in data quality, which was achieved by leveraging the retention and selectivity of a guard cartridge column. The Raptor HILIC-Si guard cartridge column provided a good chromatographic separation for all 35 analytes and 16 internal standards, mitigated matrix interferences, and significantly increased signal-to-noise ratios and signal intensities.
This method has been developed for research use only; it is not suitable for use in diagnostic procedures without further evaluation.

