Influencing Selectivity in LC-MS Peptides Analysis on the Raptor ARC-18 Column
Abstract
Methods for LC-MS peptide analysis often use acidified mobile phases, which can alter retention and selectivity. Here, we demonstrate the effects of modifying acid type, acid concentration, temperature, and gradient slope using several test probes. A Raptor ARC-18 column was employed for this work because the stationary phase ligand is sterically protected, making it extremely stable and resistant to acid damage at low pHs, which is an important characteristic when developing LC-MS methods for peptide analysis.
Introduction
With the influx of biotherapeutics in medical research and healthcare, LC-MS analysis of peptides and small proteins continues to grow. Many of these analyses utilize acid-modified mobile phases to improve peak shape [1]; however, the effects of acidic mobile phases on selectivity and retention are often not well understood. In this article, we will explore the effects of acid type, acid concentration, temperature, and gradient slope on the selectivity and retention of several peptide probes and one small protein probe (cytochrome C) using the Raptor ARC-18 LC column. This column was selected because the ligand is sterically protected from acid damage and is stable to pH 1.0. In addition, it contains superficially porous particles (SPP), which allow for faster analysis times than can be achieved with fully porous particle (FPP) columns.
Figure 1: Raptor ARC-18 LC Column Properties.
Column Description
|
Stationary Phase Category: Ligand Type: Particle: Pore Size: Surface Area: |
Recommended Usage: Properties:
|
Experimental
Several experiments were carried out to demonstrate the effects of acid-modified mobile phases, temperature, and gradient slope on LC-MS peptide analysis. For this work, the peptide and small protein standards in Table I and a bovine serum albumin (BSA) trypsin digest standard (Protea) were diluted in acidified water. These standards were injected into a Waters ACQUITY UPLC H-Class system configured with a PDA and QDa for simultaneous UV and MS detection. A wavelength of 215 nm and electrospray ionization in positive ion mode was used for UV and MS detection, respectively. Reversed-phase separations were performed using water and acetonitrile mobile phases modified with 0.01, 0.05, and 0.1% formic acid (FA) or trifluoroacetic acid (TFA) under gradient conditions using a Restek Raptor ARC-18 LC column. Specific conditions for each experiment are presented in Figures 2-5.
Table I: Probes used for LC-MS Peptide Analyses
|
Peak ID |
Compound |
Molecular Weight (Da) |
SIR (m/z) |
|
1 |
GLY-TYR |
238 |
239.3 |
|
2 |
VAL-TYR-VAL |
380 |
380.5 |
|
3 |
MET-ARG-PHE-ALA |
524 |
524.6 |
|
4 |
Methionine enkephalin |
574 |
574.6 |
|
5 |
Leucine enkephalin |
556 |
556.7 |
|
6 |
Angiotensin II |
1,046 |
524.4 |
|
7 |
Neurotensin |
1,700 |
837.9 |
|
8 |
Cytochrome C |
12,300 |
1,020.6 |
Results and Discussion
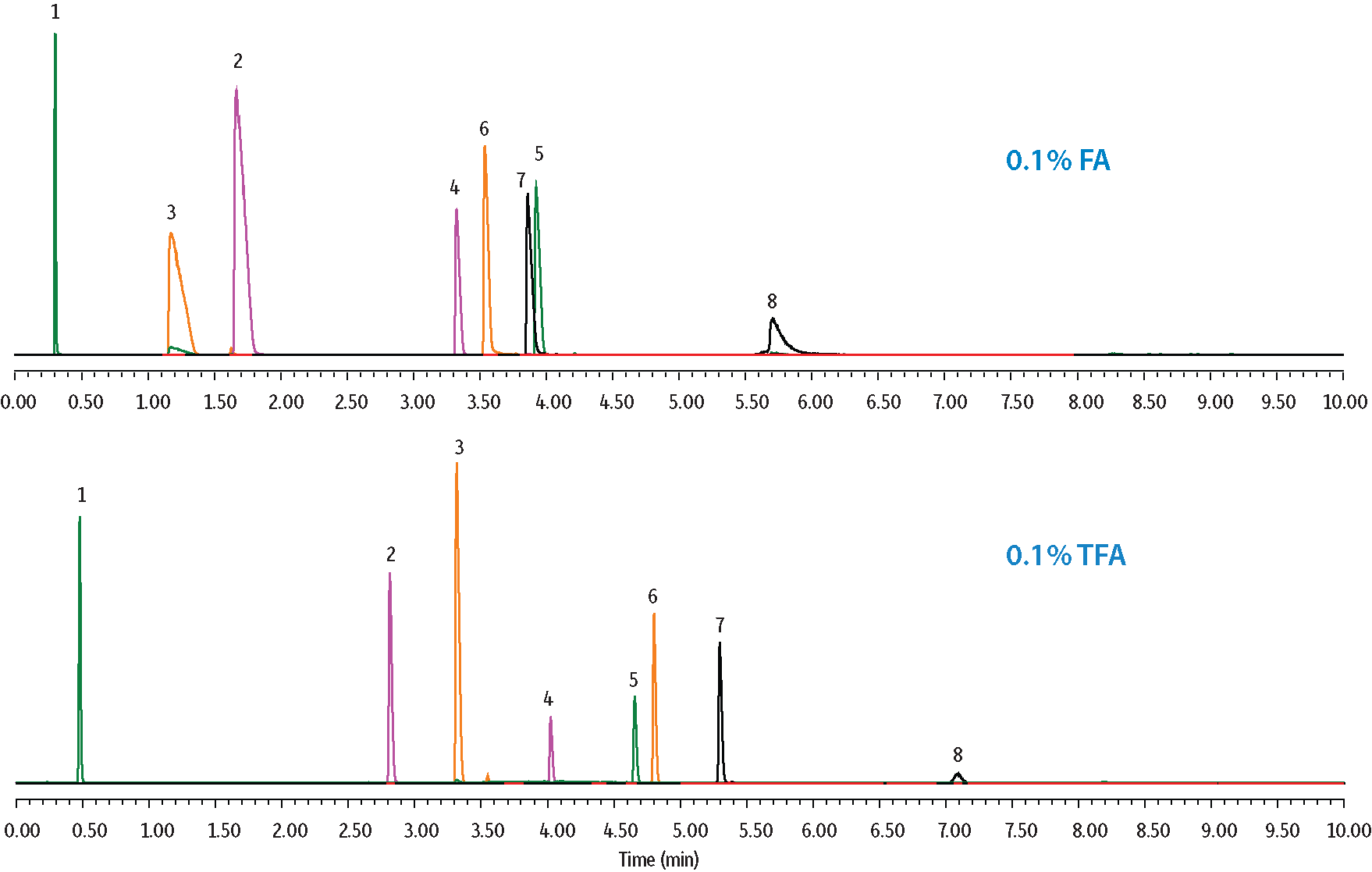
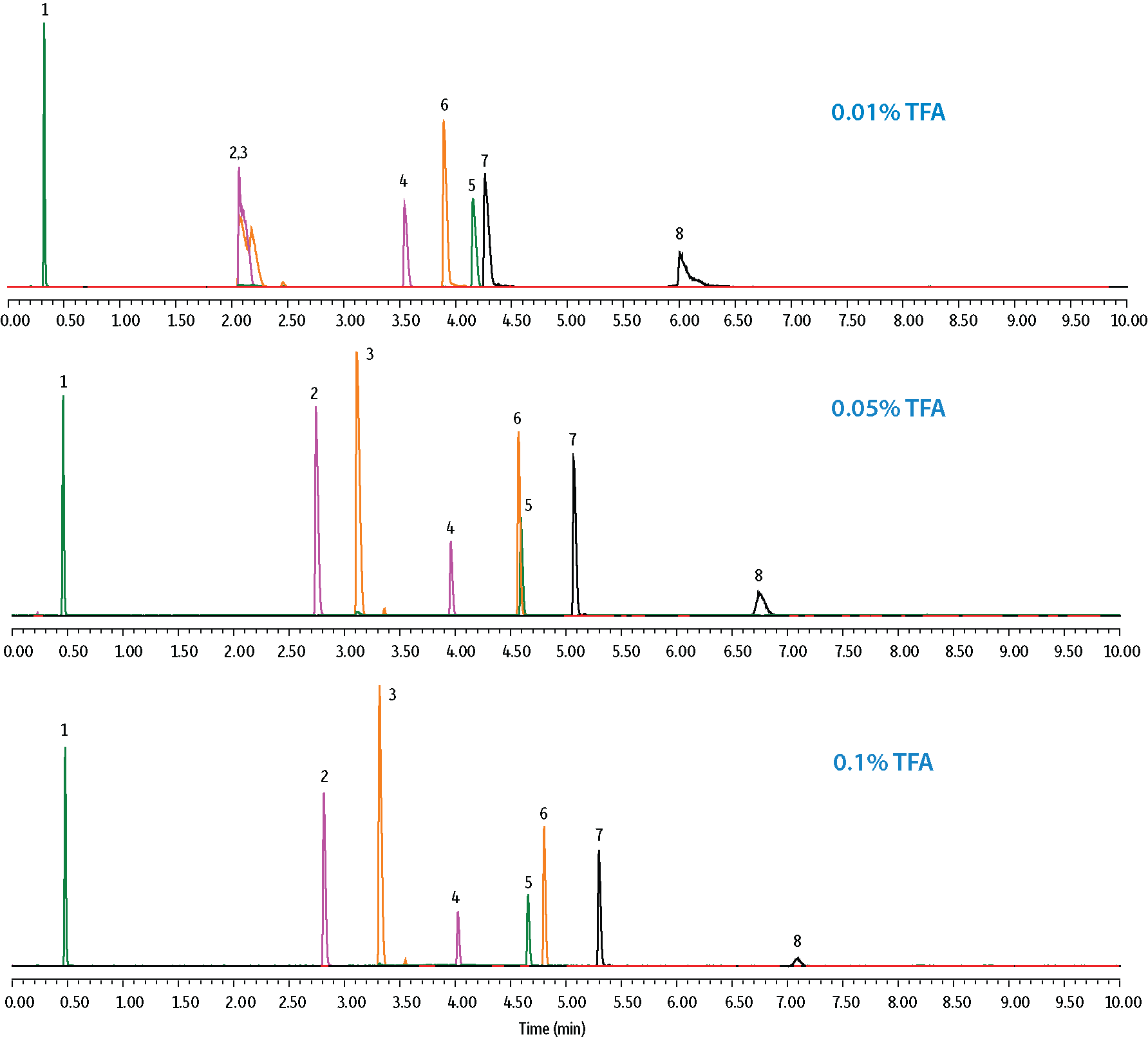
Several probes were used to aid in the understanding of selectivity changes that affect LC-MS peptide analyses when the type and concentration of the acidic modifier in the mobile phase is manipulated. The retention of peptides containing basic amino acids (e.g., arginine) exhibited considerably large changes in retention when switching between formic acid and TFA. In Figure 2, the peptide MET-ARG-PHE-ALA not only changed elution order but also displayed improved peak shape and was more retained due to the increased ion pairing capabilities of TFA over formic acid. Alternatively, VAL-TYR-VAL is a polar peptide with an overall neutral charge whose changes in selectivity result from the favorable decrease in mobile phase pH when TFA is used; in this case, the peptide was less ionized and therefore better retained. Variations in acid concentration had a comparable effect. In Figure 3, overall retention times increased with increasing concentrations of TFA, and improvements in peak shape were observed for the small protein, cytochrome C (12.3 kDa). For this reason, sterically protected columns that are stable at low pH, such as the Raptor ARC-18 column, are often preferred for LC-MS peptide analysis.
Figure 2: Effects of Acid Type on Selectivity and Peak Shape.
| Column | Raptor ARC-18 (cat.# 9314A12) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | Custom peptide/small protein standard mix | ||||||||||||||||||||||||
| Diluent: | 0.1% Formic acid in water | ||||||||||||||||||||||||
| Conc.: | 0.2–0.4 mg/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water + acid | ||||||||||||||||||||||||
| B: | Acetonitrile + acid | ||||||||||||||||||||||||
|
| Detector | MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | SRM |
| Instrument | UHPLC |
Figure 3: Effects of Acid Concentration on Retention and Selectivity.
| Column | Raptor ARC-18 (cat.# 9314A12) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Temp.: | 60 °C | ||||||||||||||||||||||||
| Standard/Sample | Custom peptide/small protein standard mix | ||||||||||||||||||||||||
| Diluent: | Water + 0.1% formic acid | ||||||||||||||||||||||||
| Conc.: | 0.2–0.4 mg/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water + TFA | ||||||||||||||||||||||||
| B: | Acetonitrile + TFA | ||||||||||||||||||||||||
|
| Detector | MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MS scan |
| Instrument | UHPLC |
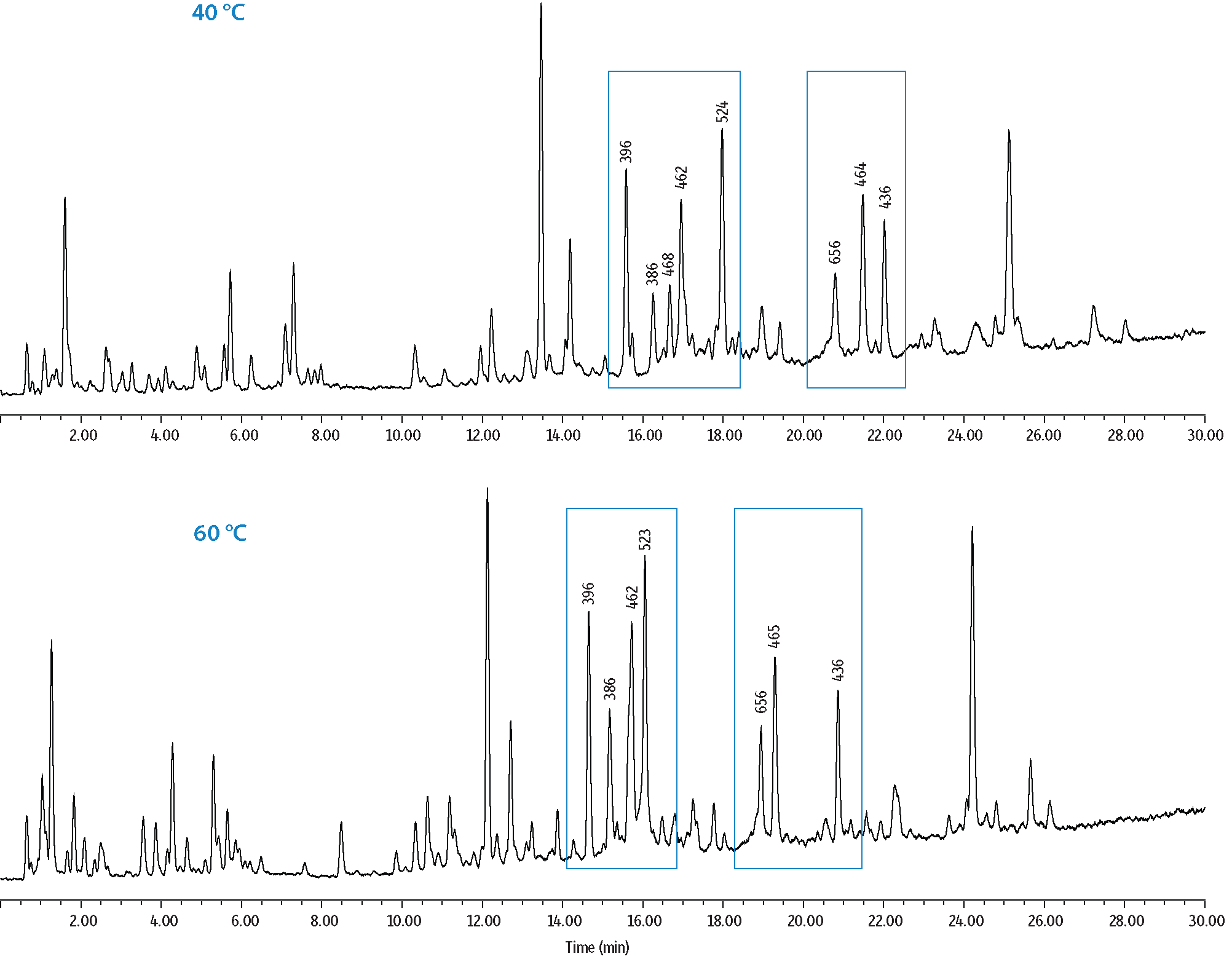
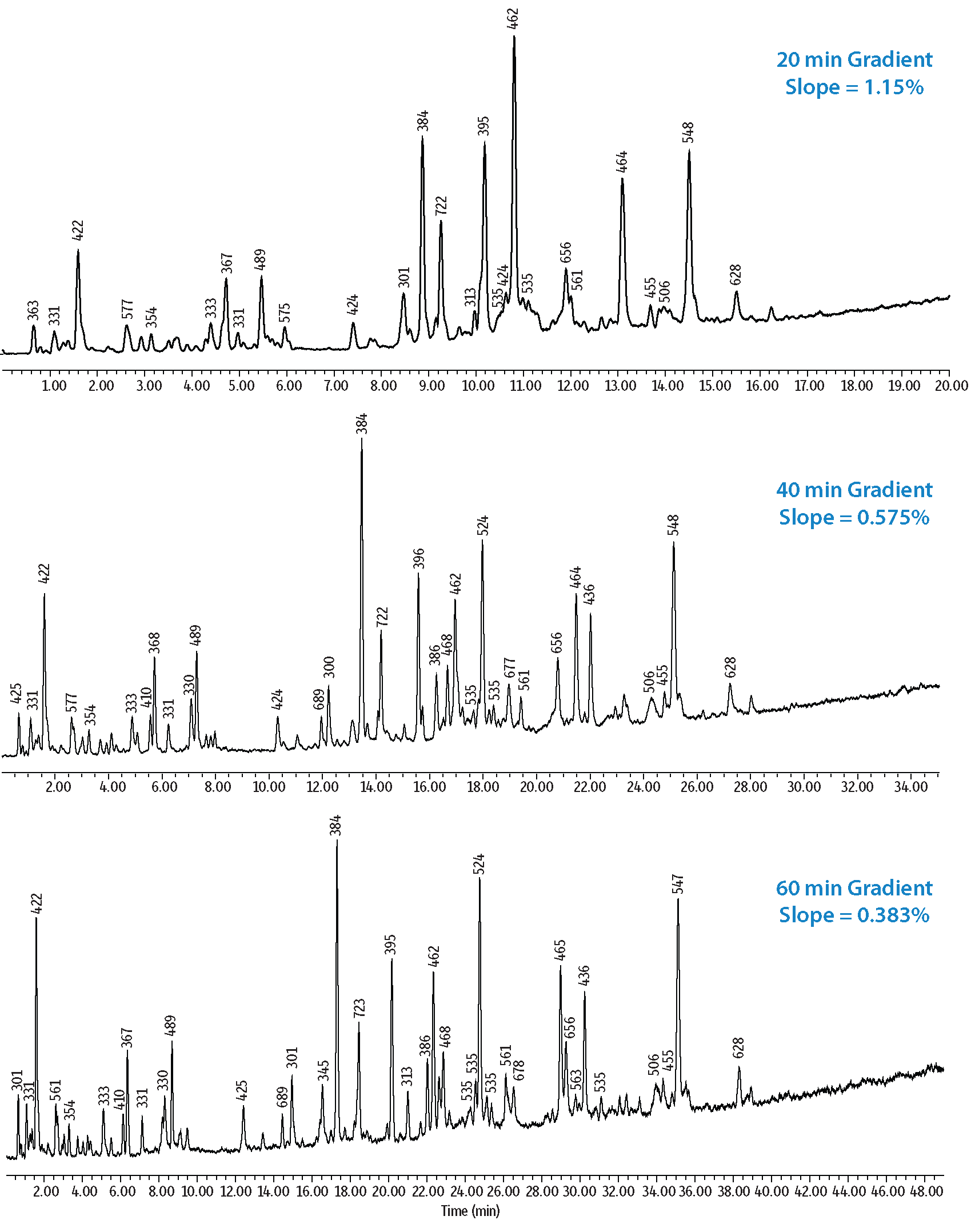
In a second set of experiments, resolution and elution order changes were examined when temperature and gradient modifications were made to the analysis of a bovine serum albumin (BSA) trypsin digest standard. In the resulting peptide maps shown in Figure 4, base peak mass 468 m/z was resolved from an adjacent peak when a lower column oven temperature (40 °C) was used. Similarly, the gradient slope was shown to impact the band spacing of closely eluting compounds. This is demonstrated in Figure 5 where the number of resolved peaks increased with decreasing gradient slope. In general, steeper gradients can be used for the separation of specific peptides in order to achieve short cycle times. However, if numerous peptides from a digest are to be analyzed, a shallower gradient is typically preferred.
Figure 4: Effects of Temperature on the Selectivity of Peptides in a BSA Tryptic Digest Standard.
| Column | Raptor ARC-18 (cat.# 9314A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Standard/Sample | Bovine serum albumin (BSA) tryptic digest standard | ||||||||||||||||||||
| Diluent: | Water + 0.1% formic acid | ||||||||||||||||||||
| Conc.: | 5 pmol | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water + 0.1% formic acid | ||||||||||||||||||||
| B: | Acetonitrile + 0.1% formic acid | ||||||||||||||||||||
|
| Detector | MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MS scan |
| Instrument | UHPLC |
Figure 5: Effects of Gradient Steepness on the Selectivity of Peptides in a BSA Tryptic Digest Standard.
| Column | Raptor ARC-18 (cat.# 9314A12) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | |||||||||||||||
| Particle Size: | 2.7 µm | |||||||||||||||
| Pore Size: | 90 Å | |||||||||||||||
| Temp.: | 40 °C | |||||||||||||||
| Standard/Sample | Bovine serum albumin (BSA) tryptic digest standard | |||||||||||||||
| Diluent: | Water + 0.1% formic acid | |||||||||||||||
| Conc.: | 5 pmol | |||||||||||||||
| Inj. Vol.: | 2 µL | |||||||||||||||
| Mobile Phase | ||||||||||||||||
| A: | Water + 0.1% formic acid | |||||||||||||||
| B: | Acetonitrile + 0.1% formic acid | |||||||||||||||
|
| Detector | MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MS scan |
| Instrument | UHPLC |
Conclusion
Relatively small changes in chromatographic parameters can have a large impact on LC-MS peptide analysis. In this work, we demonstrated that significant shifts in selectivity and retention can occur in response to relatively small changes to mobile phase acid (type and concentration), temperature, and gradient slope. Sterically protected columns like the Raptor ARC-18 column are stable at low pH and provide the versatility required for the development of peptide applications.

