Method for the Simultaneous Analysis of Methionine Pathway Metabolites and Methylmalonic Acid
Abstract
This rapid, 4-minute LC-MS/MS method allows simultaneous quantitative analysis of total homocysteine and methylmalonic acid without the need for derivatization or additional amino acid testing. Full chromatographic separation of homocysteine, methionine, cysteine, cystathionine, methylmalonic acid, and succinic acid in plasma was also achieved.
Introduction
Cobalamin, also known as vitamin B12, plays an essential role in many metabolic processes, including DNA synthesis, amino acid metabolism, and energy production. Cobalamin disorders can occur if these processes are disrupted by either genetic mutations or a deficiency in B vitamins, such as cobalamin (B12); folic acid (B9); or pyridoxine (B6). Clinical diagnosis of certain cobalamin disorders is based on critical metabolites in the methionine pathway as well as on methylmalonic acid. In particular, assays of total homocysteine, methionine, and methylmalonic acid in plasma are used for differential diagnosis. Immunoassays and LC-MS/MS methods are used for the analysis of methionine pathway metabolites and methylmalonic acid, but both have limitations. Immunoassay is not as sensitive as LC-MS/MS analysis, but with LC-MS/MS, a lengthy and time-consuming derivatization is often needed during sample preparation.
Homocysteine is an endogenous, sulfur-containing amino acid that is formed during the metabolism of methionine to cysteine. It is irreversibly catabolized by transsulfuration to cysteine or remethylated to methionine with the aid of B vitamins [1]. It is an important biomarker for homocystinuria, which is caused by a defect in methionine pathway enzymes and results in the accumulation of both methionine and homocysteine in blood and urine. It is also used to diagnose hyperhomocysteinemia, which is caused by a deficiency in vitamin B12 or B9 and characterized by elevated levels of homocysteine in plasma [2,3].
In plasma, homocysteine occurs primarily in oxidized disulfide forms. Less than 1% occurs as the free reduced homocysteine monomer, whereas 80–90% occurs as a disulfide bound to plasma proteins, and the remaining 10-20% is found as either homocysteine-cysteine mixed disulfide or as homocystine (homocysteine dimer). The sum of these four forms is referred to as total plasma homocysteine, and it is the parameter used for diagnosis [4]. To convert all the oxidized disulfide forms of homocysteine to the free monomer form, dithiothreitol (DTT) can potentially be used as a sulfhydryl reducing agent (Figure 1).
Figure 1: In this method for methionine pathway metabolites, dithiothreitol is used to reduce the disulfide forms of homocysteine (such as the homocystine dimer form) into free homocysteine monomer so that total homocysteine can be reported.
Methylmalonic acid (MMA) is a small, water-soluble organic acid produced during normal metabolic processes. It can be used as a biomarker for methylmalonic acidemia and megaloblastic anemia. Methylmalonic acid also has a naturally occurring isomer, succinic acid (Figure 2), which can be present and interfere with analysis. These isomers must be chromatographically separated to ensure accurate quantitative methylmalonic acid analysis.
Figure 2: Methylmalonic acid and succinic acid are isomers that must be chromatographically separated to ensure accurate results.
In this study, our goal was to develop a simple, fast analysis of an expanded biomarker panel for cobalamin disorders that does not require derivatization. The LC-MS/MS method detailed here utilizes a straightforward DTT-based sample preparation and provides baseline separation and positive identification of homocysteine, methionine, cysteine, cystathionine, methylmalonic acid, and succinic acid in plasma. In addition, it produces accurate quantitative results for total plasma homocysteine and methylmalonic acid.
Experimental
Plasma Samples
Plasma samples (100 µL) were added to microcentrifuge tubes along with 5 µL of internal standard (5 ng/mL DL-homocysteine-d4 and MMA-D3) followed by 20 µL of 0.5 M dithiothreitol (DTT) to serve as a reducing agent. Next, the samples were vortexed for 10 seconds. The samples were then incubated at room temperature (20 oC) and protected from light for 30 minutes. After 30 minutes, 300 µL of methanol was added. The samples were then vortexed again for 10 seconds and centrifuged for 10 minutes at 4000 rpm. The supernatant was pipetted into a vial containing a 250 µL vial insert and injected for LC-MS/MS analysis.
Calibrators and Quality Control Samples
Phosphate buffered saline (PBS) was used as a matrix surrogate due to the endogenous analyte levels found in plasma. PBS was diluted to a 1:9 concentration with HPLC water to obtain a 10x PBS solution. This solution was used to prepare calibration and QC standards.
While homocysteine, homocystine, cysteine, cystathionine, methionine, methylmalonic acid, and succinic acid were all analyzed by this method in plasma to assess chromatographic performance and method suitability for sample screening, only homocysteine and methylmalonic acid were evaluated quantitatively. For quantitative analysis, homocysteine (monomer) and methylmalonic acid standards were prepared in PBS across a range of 25–5000 ng/mL for homocysteine and 10–5000 ng/mL for methylmalonic acid, and then 100 µL aliquots were mixed with 5 µL of internal standard (5 µg/mL DL-homocysteine-d4 and MMA-D3). Calibrators and quality control samples were then subjected to the same DTT-based sample preparation procedure used for the plasma samples.
Instrument Conditions
For this LC-MS/MS method for methionine pathway metabolites and methylmalonic acid analysis, samples were run on a Shimadzu Nexera X2 coupled with a SCIEX 4500 mass spectrometer. The chromatographic conditions are detailed below, and the retention times, MRM transitions, and analysis mode for each analyte are provided in Table I.
| Column: | Raptor Polar X (2.7 µm, 50 mm x 2.1 mm ID [cat.# 9311A52]) | |
| Guard column: | Raptor Polar X EXP guard column cartridge (2.7 µm, 5 mm x 2.1 mm [cat.# 9311A0252]) | |
| Column temp.: | 40 °C | |
| Injection volume: | 5 µL | |
| Mobile phase A: | Water, 0.5% formic acid | |
| Mobile phase B: | Acetonitrile | |
| Time (min) | %B | |
| 0.00 | 85 | |
| 1.00 | 55 | |
| 3.00 | 10 | |
| 3.10 | 85 | |
| 4.00 | stop | |
| Flow rate: | 0.6 mL/min | |
| Ion mode: | Positive and negative ESI | |
Table I: Analyte Retention Times, Transitions, and Ion Modes
|
Analyte |
Retention Time (min) |
Precursor Ion |
Product Ion 1 |
Product Ion 2 |
Polarity |
|
L-Methionine |
0.75 |
150.0 |
104.1 |
- |
+ |
|
DL-Homocysteine-D4 |
0.79 |
140.1 |
94.1 |
- |
+ |
|
L-Homocysteine |
0.81 |
136.2 |
90.1 |
- |
+ |
|
L-Cysteine |
0.97 |
122.1 |
76.0 |
- |
+ |
|
L-Homocystine |
1.29 |
268.9 |
136.0 |
- |
+ |
|
Succinic acid |
1.32 |
117.0 |
72.9 |
55.1 |
- |
|
DL-Cystathionine |
1.45 |
221.3 |
119.8 |
113.9 |
- |
|
Methylmalonic acid-D3 |
2.91 |
120.1 |
58.0 |
75.9 |
- |
|
Methylmalonic acid |
2.95 |
117.0 |
55.1 |
72.9 |
- |
Results and Discussion
Optimization of DTT
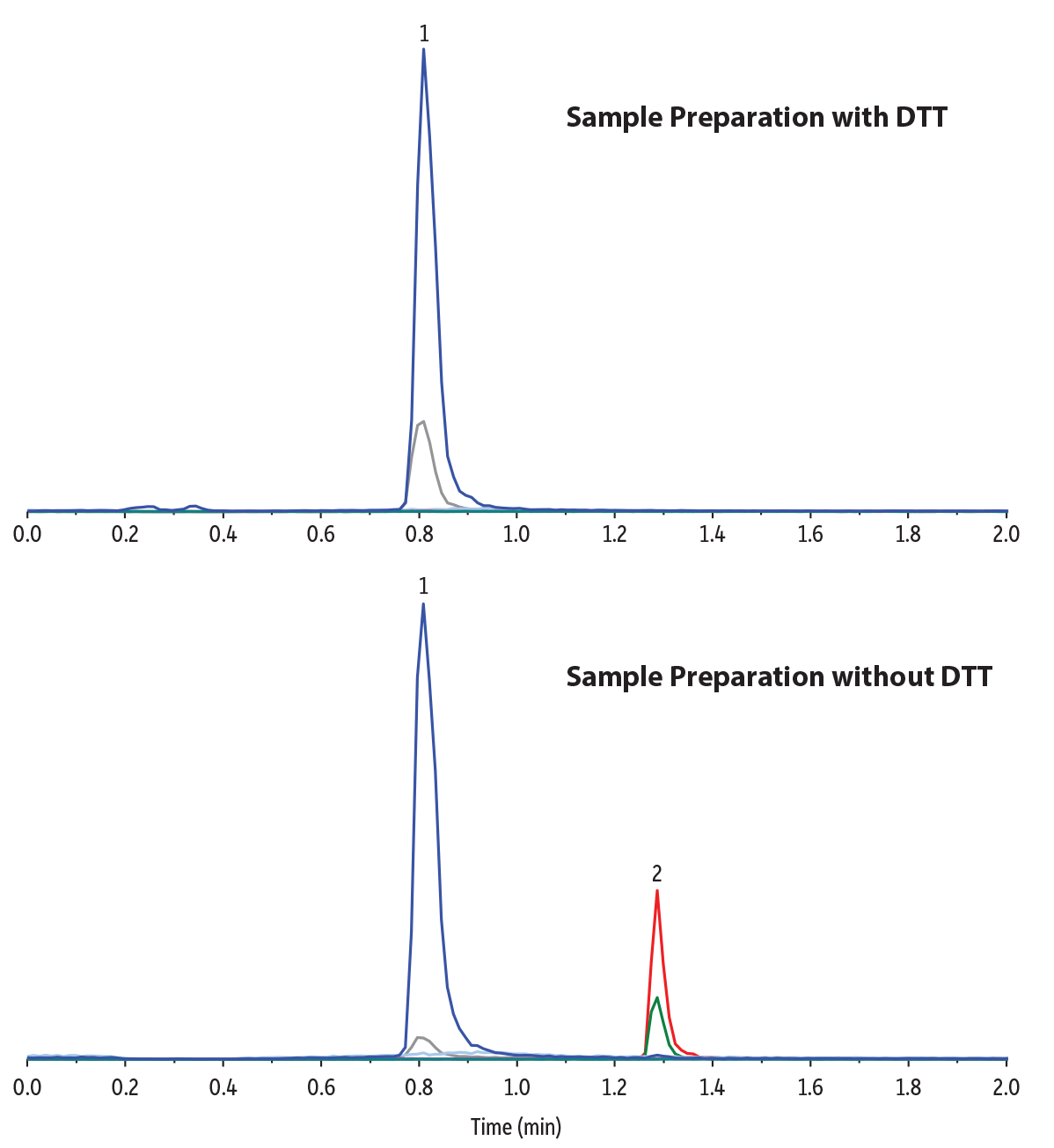
Preliminary, nonquantitative experiments were conducted to evaluate the effectiveness of using dithiothreitol (also referred to as DTT or Cleland’s reagent) as a reducing agent to convert all forms of homocysteine into the free monomer. In this study, 20 µL of 0.5 M DTT was added to 100 µL plasma samples that were fortified with both homocystine (dimer) and homocysteine (monomer) at 100 ng/mL. The samples were incubated at room temperature (around 20 oC) and protected from light for 10, 20, 30, 40, 50, or 60 minutes and then precipitated with 300 µL methanol, vortexed for 10 seconds, centrifuged for 10 minutes, and analyzed by LC-MS/MS. It was found that a 30-minute incubation time was enough to obtain the maximum recovery of homocysteine (Figure 3). Longer incubation times, while not deleterious to the results, only increased sample preparation time. Figure 4 chromatographically compares the sample preparation with and without the use of DTT and demonstrates that DTT completely converts the homocystine dimer into homocysteine monomer.
Figure 3: Effect of DTT Incubation Time on Homocysteine Recovery
Figure 4: A simple DTT sample preparation effectively converts all homocystine (dimer) to homocysteine (monomer) without time-consuming derivatization.
| Column | Raptor Polar X (cat.# 9311A52) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Polar X EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9311A0252) | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.5% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
|
| Detector | SCIEX 4500 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | With DTT A 100 ng/mL standard mix of homocysteine (monomer) and homocystine (dimer) was prepared in plasma. A 100 μL aliquot was taken from the standard and mixed with 20 μL of 0.5 M dithiothreitol (DTT). The sample was vortexed for 10 seconds and then left to incubate at room temperature in darkness for 30 minutes. After 30 minutes, 300 μL of methanol was added, and the sample was vortexed for 10 seconds and then centrifuged for 10 minutes at 4000 rpm. 100 μL of the supernatant was added to a 2 mL vial (cat.# 21142) containing a vial insert (cat. # 21776) and capped with a short screw cap (cat.# 24498). Without DTT A 100 ng/mL standard mix of homocysteine (monomer) and homocystine (dimer) was prepared in plasma. A 100 μL aliquot was taken from the standard and was vortexed for 10 seconds. After vortexing, 300 μL of methanol was added, and the sample was vortexed for 10 seconds and then centrifuged for 10 minutes at 4000 rpm. 100 μL of the supernatant was added to a 2 mL vial (cat.# 21142) containing a vial insert (cat. # 21776) and capped with a short screw cap (cat.# 24498). |
Chromatographic Performance
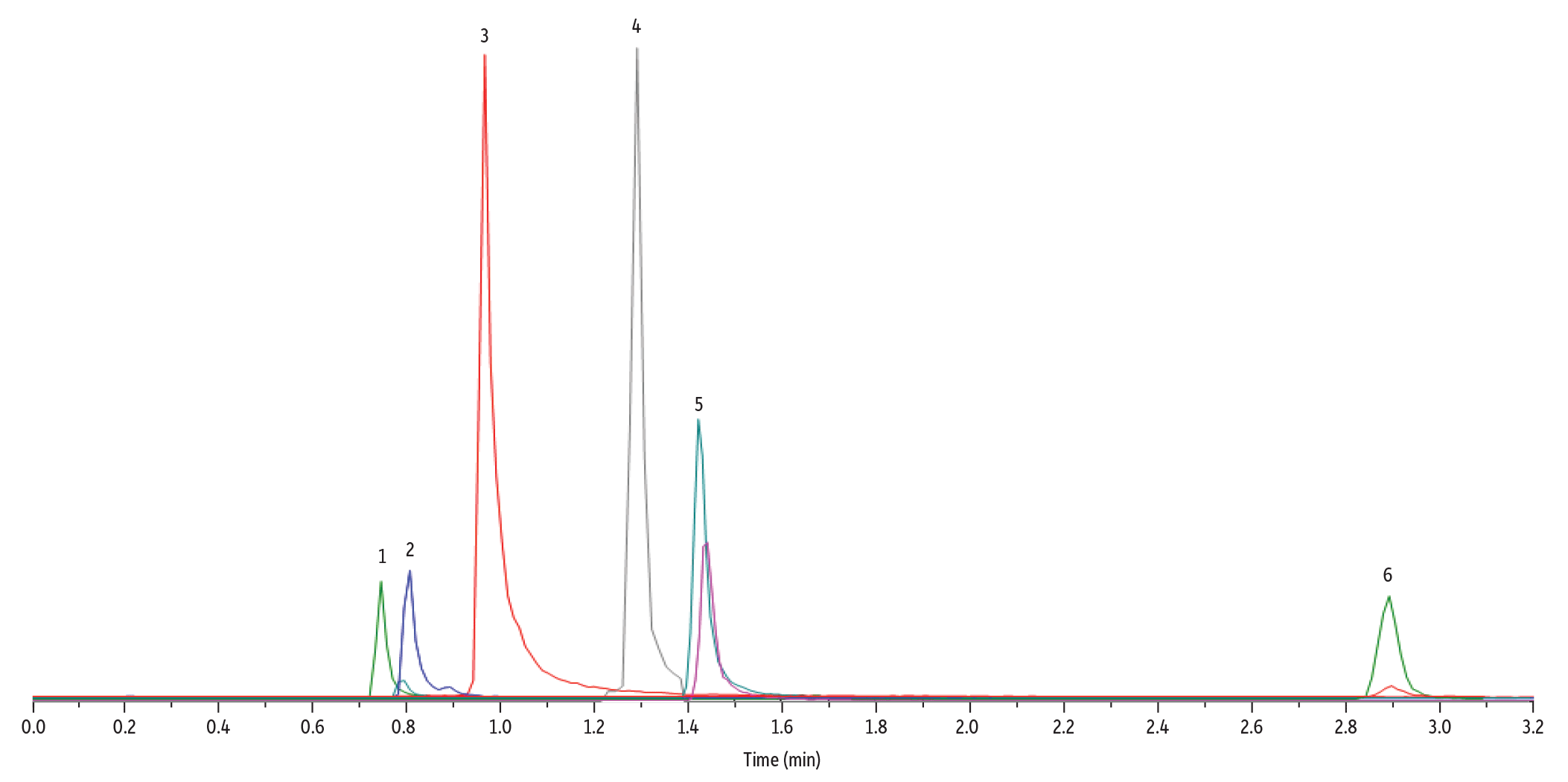
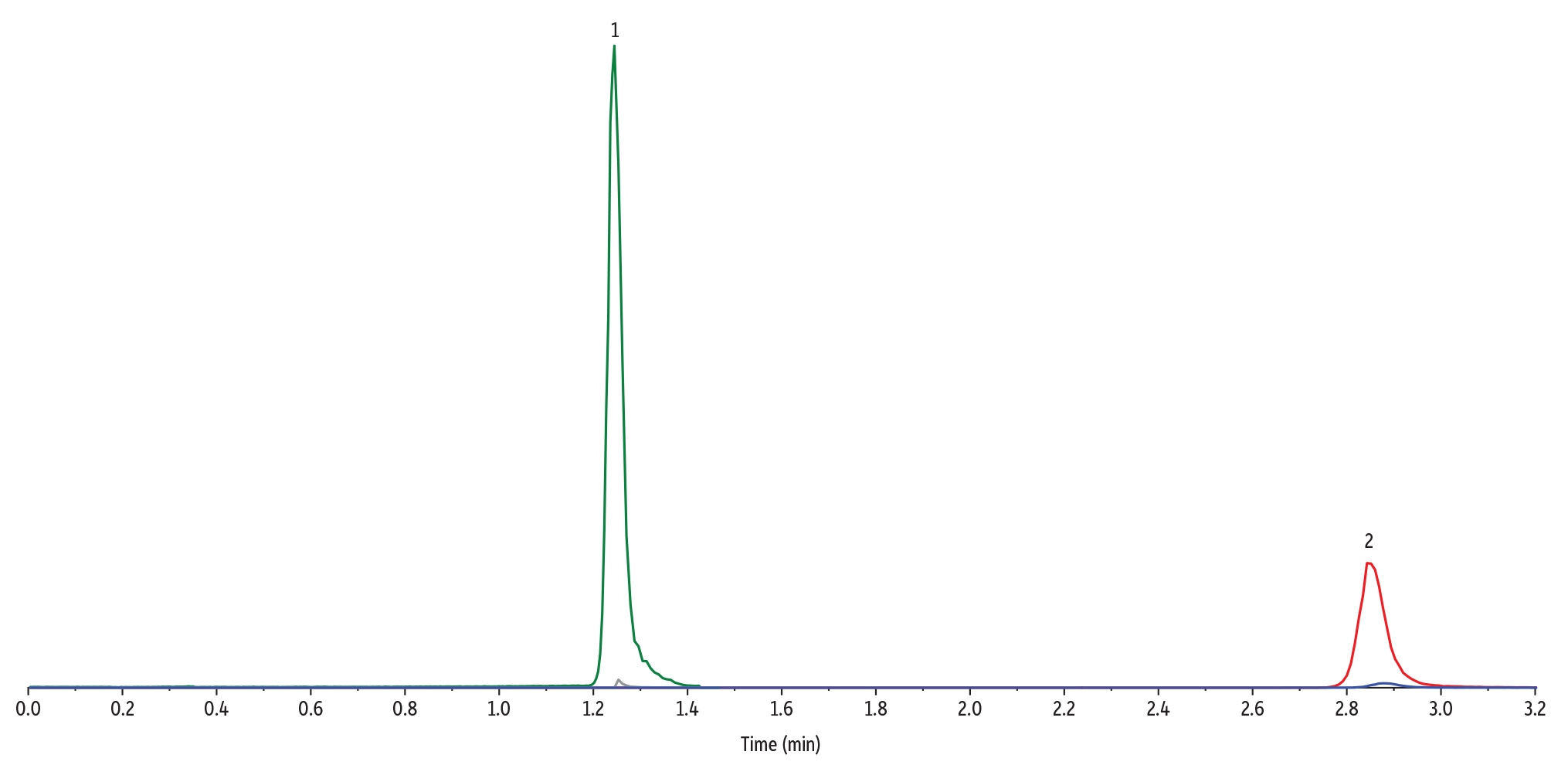
For this LC-MS/MS method for methionine pathway metabolites and methylmalonic acid analysis, the chromatographic separation of all target analytes can be seen in Figure 5. Most notably, the method achieved complete separation of methylmalonic acid and its naturally occurring isomer, succinic acid (Figure 6). This isomeric separation is important for accurate quantitation of methylmalonic acid because the compounds share the same MRM transitions. The use of a Raptor Polar X column, with its hydrophilic interaction liquid chromatography (HILIC) and ion-exchange capabilities, allowed full separation of these critical analytes, which preliminary experiments showed was not easily achievable on other stationary phases.
Figure 5: Chromatographic separation of all target analytes was achieved using a Raptor Polar X column under HILIC conditions.
| Column | Raptor Polar X (cat.# 9311A52) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Polar X EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9311A0252) | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.5% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
|
| Detector | SCIEX 4500 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+/ESI- |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | A 100 ng/mL standard mix of all of the analytes in this method was prepared in plasma. A 100 µL aliquot was taken from the standard and mixed with 20 µL of 0.5 M dithiothreitol (DTT). The sample was vortexed for 10 seconds and then left to incubate at room temperature in darkness for 30 minutes. After 30 minutes, 300 µL of methanol was added, and the sample was vortexed for 10 seconds, and then centrifuged for 10 minutes at 4000 rpm. 100 µL of the supernatant was added to a 2 mL vial (cat.# 21142) containing a vial insert (cat. # 21776) and capped with a short screw cap (cat.# 24498). |
Figure 6: A Raptor Polar X column provides complete separation of methylmalonic acid and succinic acid isomers.
| Column | Raptor Polar X (cat.# 9311A52) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor Polar X EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9311A0252) | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 0.5% formic acid | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
|
| Detector | SCIEX 4500 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | A 100 ng/mL standard mix of methylmalonic acid and succinic acid was prepared in plasma. A 100 µL aliquot was taken from the standard and mixed with 20 µL of 0.5 M dithiothreitol (DTT). The sample was vortexed for 10 seconds and then left to incubate at room temperature in darkness for 30 minutes. After 30 minutes, 300 µL of methanol was added, and the sample was vortexed for 10 seconds and then centrifuged for 10 minutes at 4000 rpm. 100 µL of the supernatant was added to a 2 mL vial (cat.# 21142) containing a vial insert (cat. # 21776) and capped with a short screw cap (cat.# 24498). |
Linearity
Linearity was demonstrated using a 1/x2 weighted linear regression, and both homocysteine and methylmalonic acid showed acceptable R2 values. The R2 value for homocysteine was 0.9992, and the R2 average for the two transitions of methylmalonic acid was 0.9969. The linear ranges were 10–5000 ng/mL for methylmalonic acid and 25–5000 ng/mL for homocysteine.
Accuracy and Precision
Accuracy and precision were evaluated using two sets of QC samples that were analyzed over a total of three days, and the results are presented in Table II. Method accuracy was demonstrated by recovery values being within 15% of the nominal concentrations for all QC samples. Precision was shown by the %RSD values being ≤13% for all QC samples. Note that different low QC levels were prepared for methylmalonic acid and homocysteine based on their respective linear ranges (in Table II, “NA” indicates which concentration is not applicable for each analyte).
Table II: Interday Accuracy and Precision of Quality Control Samples in PBS
| Total Plasma Homocysteine | Methylmalonic Acid | |||||
|
Avg Conc. (ng/mL) |
Avg. Accuracy (%) |
% RSD |
Avg Conc. (ng/mL) |
Avg. Accuracy (%) |
% RSD |
|
|
QC 25 ng/mL |
NA |
24.2 |
95.2 |
11.0 |
||
|
QC 50 ng/mL |
47.6 |
94.7 |
13.0 |
NA | ||
|
QC 150 ng/mL |
152.7 |
103.2 |
5.8 |
158.0 |
105.3 |
10.9 |
|
QC 600 ng/mL |
597.7 |
99.6 |
2.9 |
583.3 |
97.4 |
5.4 |
|
QC 1500 ng/mL |
1540.0 |
102.6 |
5.3 |
1483.3 |
99.1 |
5.5 |
|
QC 3000 ng/mL |
3183.3 |
105.7 |
2.8 |
3170.0 |
105.6 |
10.5 |
Accuracy of Surrogate Matrix: Standard Addition Testing
Standard addition was used in this method due to the presence of endogenous homocysteine and methylmalonic acid in the plasma samples. First, blank lots of plasma were treated with DTT and tested against the calibration curve to quantify the endogenous levels and to help determine an appropriate concentration for standard addition spiking. Then, to test the quantitative accuracy of the method as well as the accuracy of using PBS as a surrogate matrix, plasma samples were spiked with methylmalonic acid and homocystine (dimer) at 1000 ng/mL. Five samples each from four lots were prepared and tested (n = 20).
The results showed acceptable accuracy and intraday repeatability for both methylmalonic acid and homocysteine, with a % difference from the expected value of less than 15% and low %RSD values for all lots of plasma (Tables III and IV). This evaluation also demonstrated that DTT worked efficiently in converting the homocystine dimer into the homocysteine monomer because the samples were fortified with the dimer form and the calibrators were made with the monomer form.
Table III: Intraday Methylmalonic Acid Repeatability for Standard Addition Samples.
|
Plasma Lot |
Expected |
Average |
% Difference |
%RSD |
|
Lot 1 |
1008.3 |
907.2 |
10.0 |
3.8 |
|
Lot 2 |
1055.4 |
946.6 |
10.3 |
12.0 |
|
Lot 3 |
1304.0 |
1141.2 |
12.5 |
10.1 |
|
Lot 4 |
1000.0 |
1142.0 |
14.2 |
5.5 |
Table IV: Intraday Homocysteine Repeatability for Standard Addition Samples.
|
Plasma Lot |
Expected |
Average |
% Difference |
%RSD |
|
Lot 1 |
2506.1 |
2606.0 |
4.0 |
7.5 |
|
Lot 2 |
2666.0 |
2902.4 |
8.9 |
3.2 |
|
Lot 3 |
3734.2 |
3570.1 |
4.4 |
8.5 |
|
Lot 4 |
3620.1 |
3320.3 |
8.3 |
1.4 |
Column Robustness
Column robustness was also evaluated for this LC-MS/MS method for methionine pathway metabolites and methylmalonic acid analysis by running more than 700 matrix injections on the same Raptor Polar X column. Highly consistent retention times between the first and last injections for all analytes demonstrated very stable column performance (Table V).
Table V: Consistent Retention Times Demonstrate Column Robustness
|
Analyte |
Inj. 1 |
Inj. 700 |
% Diff |
|
L-Methionine |
0.76 |
0.78 |
2.50 |
|
DL-Homocysteine-D4 |
0.78 |
0.80 |
2.56 |
|
L-Homocysteine |
0.80 |
0.82 |
2.50 |
|
L-Cysteine |
0.95 |
0.97 |
2.10 |
|
Succinic Acid |
1.33 |
1.30 |
2.27 |
|
DL-Cystathionine |
1.45 |
1.41 |
2.72 |
|
Methylmalonic acid-D3 |
2.91 |
2.83 |
2.74 |
|
Methylmalonic acid |
2.95 |
2.86 |
3.05 |
Conclusion
A rapid 4-minute LC-MS/MS method was developed for the simultaneous analysis of methionine pathway metabolites, including homocysteine, methionine, cysteine, and cystathionine, as well as methylmalonic acid and succinic acid in plasma. Complete chromatographic separation of all compounds was achieved using a Raptor Polar X column under HILIC conditions. The method employed a simple sample reduction procedure using DTT instead of time-consuming derivatization procedures. Reproducible retention times and selectivity were demonstrated over the course of testing for all analytes, which may be beneficial for sample screening. The method also produced acceptable precision and accuracy for total plasma homocysteine and methylmalonic acid, indicating that with validation it could be an effective quantitative technique for these two important biomarkers.
References
- Jiang, B. Mistretta, S. Elsea, Q. Sun, Simultaneous determination of plasma total homocysteine and methionine by liquid chromatography-tandem mass spectrometry, Clinica Chemica Acta, 464 (2017) 93-97. DOI: 10.1016/j.cca.2016.11.017
- Bártl, P. Chrastina, J. Krijt, J. Hodík, K. Pešková, V. Kožich, Simultaneous determination of cystathionine, total homocysteine, and methionine in dried blood spots by liquid chromatography/tandem mass spectrometry and its utility for the management of patients with homocystinuria, Clinica Chemica Acta, 437 (2014) 211-217. DOI: 10.1016/j.cca.2014.07.028
- Tomaiuolo, G. Vecchione, M. Margaglione, D. Pisanelli, E. Gandone, Stable-isotope dilution LC-ESI-MS/MS techniques for the quantification of total homocysteine in human plasma, Journal of Chromatography B, 877 (2009) 3292-3299. DOI: 10.1016/j.jchromb.2009.07.024
- Persichilli, J. Gervasoni, F. Lavarone, C. Zuppi, B. Zappacosta, A simplified method for the determination of total homocysteine in plasma by electrospray tandem mass spectrometry, Journal of Separation Science, 33 (20) (2010) 3119–3124. DOI: 10.1002/jssc.201000399

