Reduce Helium Consumption by 68% Using Nitrogen Purge Gas for VOCs in Water
- Save 490 mL of helium per sample by switching to nitrogen purge gas.
- Spend less money on lab gases and reduce your dependence on helium.
- Easily resolve critical Method 524.4 compounds using an Rtx-VMS column.
By now, everyone has felt the impact of the helium supply problem—even regulatory agencies. In order to help laboratories reduce helium use, EPA has revised Method 524.3 to allow for the use of nitrogen as a purge gas. Although labs have been doing this for years with other methods, substantial reductions in helium consumption can now be obtained when analyzing purgeable organic compounds in water by GC-MS using the revised method. By switching to nitrogen purge gas using Method 524.4, you can save an impressive 490 mL of helium per sample, which translates into a 68% reduction in helium consumption (Table I). Saving nearly 0.5 L of helium per sample quickly adds up to considerable cost savings and also insulates labs from the impact of fluctuating helium availability.
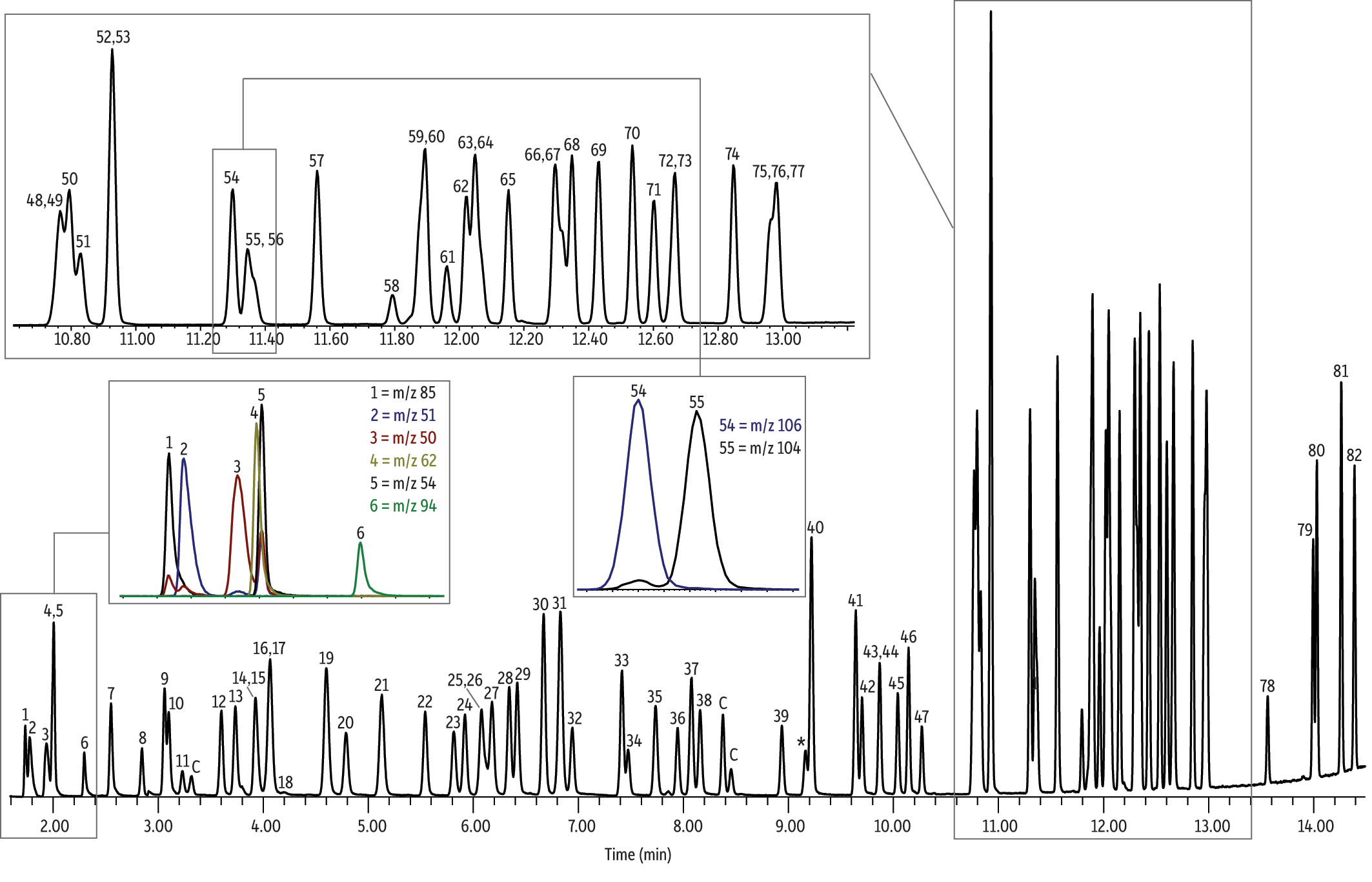
In addition to reducing helium consumption by using nitrogen purge gas, employing an Rtx-VMS column for this analysis allows all Method 524.4 criteria to be easily met. The Rtx-VMS column is recommended for purge-and-trap GC-MS analysis of VOCs by Method 524.4 because the selectivity of this column provides ample separation between all critical compounds. As shown in Figure 1, good resolution is obtained for target analytes, including o-xylene and styrene, as well as 1,1,1-trichloroethane and carbon tetrachloride. The Rtx-VMS column is listed in Method 524.4 and was used by the EPA to establish method performance [1]. No interference from overlapping peaks was observed, and the small bore 0.25 mm column results in higher column efficiency and improved separations.
Labs analyzing purgeable organic compounds in water can save money and reduce helium dependence by using Method 524.4 with nitrogen purge gas and an Rtx-VMS column.
Table I: Using nitrogen purge gas allows labs to reduce helium use by 68%.
| Instrument | Analysis Step | He Volume | N2 Volume |
| P&T | Purge | — | 440 |
| Dry Purge | — | 50 | |
| Desorb | 30.9 | — | |
| GC-MS | Split Vent* | 140 | — |
| Carrier | 13.5 | — | |
| Septum Purge | 45 | — | |
| Total Gas Volume | 719.4 | ||
| Volume of Helium Saved | 490 | ||
| Percent of Helium Saved |
68% |
*Gas saver is turned to 10 mL/min @ 1 min.
Figure 1: The Rtx-VMS column is specifically designed for separating purgeable organic compounds, which means it provides excellent resolution of the Method 524.4 VOCs analyzed here using nitrogen purge gas.
| Column | Rtx-VMS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 19915) |
|---|---|
| Standard/Sample | 524.3 Internal standard/surrogate mix (cat.# 30017) |
| 524.3 Gas calibration mix (cat.# 30014) | |
| 524.3 VOA MegaMix standard (cat.# 30013) | |
| Diluent: | RO water |
| Conc.: | 40 ng/mL (5 mL sample) |
| Injection | purge and trap split (split ratio 30:1) |
| Liner: | Premium 1.0 mm ID straight inlet liner (cat.# 23333) |
| Inj. Temp.: | 200 °C |
| Purge and Trap | |
| Instrument: | EST Encon Evolution |
| Trap Type: | Vocarb 3000 |
| Purge: | 11 min, flow 40 mL/min |
| Dry Purge: | 1 min, flow 50 mL/min |
| Desorb: | 1 min @ 260 °C, flow 30.9 mL/min |
| Bake: | 8 min @ 265 °C |
| Interface Connection: | injection port |
| Transfer Line Temp.: | 150 °C |
| Oven | |
| Oven Temp.: | 45 °C (hold 4.5 min) to 100 °C at 12 °C/min to 240 °C at 25 °C/min (hold 1.32 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 0.9 mL/min |
| Detector | MS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||||||
| Scan Program: | |||||||||||||
| |||||||||||||
| Transfer Line Temp.: | 240 °C | ||||||||||||
| Analyzer Type: | Quadrupole | ||||||||||||
| Source Temp.: | 230 °C | ||||||||||||
| Quad Temp.: | 150 °C | ||||||||||||
| Electron Energy: | 70 eV | ||||||||||||
| Solvent Delay Time: | 1.5 min | ||||||||||||
| Tune Type: | BFB | ||||||||||||
| Ionization Mode: | EI | ||||||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||||||
| Notes | Nitrogen was used as the purge gas for the EST Encon Evolution. | ||||||||||||
References
- U.S. Environmental Protection Agency, Method 524.4, Measurement of Purgeable Organic Compounds in Water by Gas Chromatography/Mass Spectrometry Using Nitrogen Purge Gas, May 2013. http://water.epa.gov/scitech/drinkingwater/labcert/upload/815R13002.pdf (accessed December 19, 2013).

