Reliable Low-Level Analysis of Glycols in Water Using Split Injection
Abstract
Obtaining consistent results for low-level analysis of glycols in water samples is difficult when using splitless injection due primarily to issues with backflash, poor peak shape, and shifting retention times. The split injection method shown here avoids these problems and reliably produces good chromatographic results. Lifetime testing demonstrated that Rtx-Wax columns produce stable results, even after column integrity was challenged with 600 water injections.
Introduction
The analysis of glycols in water is a common test performed by environmental, food safety, and chemical laboratories. Both ethylene glycol and propylene glycol have many applications across these industries because they effectively lower the freezing point of water. However, they also have a striking and significant difference: just 2–4 ounces of ethylene glycol can be fatal if ingested, whereas, propylene glycol is commonly used in foods. The toxicity of ethylene glycol and potential for exposure from a wide range of sources makes reliable low-level quantitation critical.
Exposure to glycols can occur through environmental contamination of water with automobile antifreeze, aircraft deicing liquids, and hydraulic fracturing fluid. Ethylene glycol was first used in automobile antifreeze in 1926 [1], and it is still the primary ingredient. Aircraft deicing using propylene glycol and ethylene glycol became common in the 1950s. These chemicals are also used on runways as an alternative to salt because salt corrodes landing gear components [2]. Hydraulic fracturing is another industrial application that can result in significant exposure to glycols. With this technique, pressurized fluid and sand or other solids (proppants) are used in gas drilling, which allows gas extraction from areas where it was once believed to be impossible. Both ethylene glycol and propylene glycol are common ingredients in hydraulic fracturing fluid and play a key role in stabilizing solutions and preventing emulsification [3]. Due to concern about water contamination by glycols and the other compounds that are used in hydraulic fracturing, 14 states currently require disclosure of these chemicals.
Because ethylene glycol is highly soluble in water, it is not easily concentrated by purge-and-trap or headspace methods. Therefore, aqueous splitless injection is the most frequently used sample introduction technique. However, splitless injection of glycols in water is very challenging and often produces inconsistent results due mainly to backflash, poor peak shape, and shifting retention times. Backflash occurs when the sample expansion volume exceeds the capacity of the liner. This drives the vapor cloud out of the liner, which results in poor sample transfer onto the column. Split peaks are another source of variation and occur when the glycol compounds are not focused in a narrow band but, instead, are in the condensed water that beads onto the column walls. Peak splitting is most apparent when performing a splitless injection with a polydimethylsiloxane (PDMS) phase column. These problems can prevent analysts from achieving the necessary detection limits for ethylene glycol, which are generally in the 0.5–10 ppm range.
Analyzing glycols in water samples using split injection is a much better approach because it provides fast, consistent sample transfer and reduces the amount of water injected on-column, which minimizes backflash as well as peak problems. While it may seem counterintuitive that injecting less sample can improve low-level analysis, the gains made in peak shape and reproducibility ensure reliable results even at low levels. Pairing split injection with a polyethylene glycol (PEG) analytical column is the best tactic for analyzing glycols in water because PEG stationary phases offer unique selectivity and are suitable for aqueous injections. Here, we present analytical conditions established during a long-term method development study that consistently produce good chromatographic results at low levels. We also compare the lifetime performance of widely used Rtx-Wax and Stabilwax columns using this method to determine which of these two PEG columns is ultimately most suitable for this application.
Experimental
Analytical Columns and Conditions
The performance of Rtx-Wax (cat.# 12455) and Stabilwax (cat.# 10655) columns for the analysis of glycols in water was compared using the method conditions detailed below. Over the course of a comprehensive, in-house method development study, these conditions were determined to reliably provide symmetrical peak shapes and stable retention times. In addition, the study showed that a 1 µL 50:1 split injection into a liner containing wool eliminated injection port effects, decreased backflash, and increased reproducibility. The use of wool in a 4.0 mm ID liner was critical for good reproducibility because wool allows the sample to vaporize rapidly and completely. A splitless modification of this method was used for the water injections (but not for the sample injections) during lifetime evaluation experiments in order to create more severe conditions with which to test column integrity.
| Column Format: | 30 m, 0.53 mm ID, 1.0 μm |
| Injection: | 1 μL, split 50:1 (autosampler, fast speed) |
| Liner: | Premium 4.0 mm ID Precision inlet liner w/wool (cat.# 23305.1), note that this product has been replaced by Topaz 4.0 mm ID Precision inlet liner w/wool (cat.# 23305) |
| Inj. Port Temp.: | 250 °C |
| Oven Temp.: | 40 °C (hold 1.0 min) to 250 °C at 30 °C/min |
| Carrier Gas: | He, 4 psi (constant flow) |
| Linear velocity: | 40 cm/sec |
| Detector: | FID @ 250 °C |
| Make-up Gas Flow Rate: | 45 mL/min |
| Make-up Gas Type: | N2 |
| Hydrogen flow: | 40 mL/min |
| Air flow: | 450 mL/min |
| Data Rate: | 20 Hz |
Linearity Evaluation
Ethylene glycol and propylene glycol calibration standards were prepared from a mixed glycol standard (cat.# 30471) in methanol:water (10:90). 2-Butoxy ethanol was added at a final concentration of 500 ppm in each solution (10 ppm on-column) and used as an internal standard. The standards were analyzed using split injection across a calibration range of 0.5–100 ng on-column. A calibration curve was first established on new, unused Rtx-Wax and Stabilwax columns to demonstrate initial linearity and to evaluate peak shape at different concentrations. The calibration curve was analyzed again on used columns following the 600 water injection lifetime experiment to determine if linearity and peak shape were maintained or if they had degraded.
Lifetime Testing
A lifetime study was conducted on three different lots of Rtx-Wax columns and two different lots of Stabilwax columns to determine which would better withstand repeated water injections and still maintain chromatographic performance. The experimental design consisted of 10 injections of water (1 µL splitless) followed by an injection of a 50 µg/mL glycol standard (50:1 split injection, 1 ng on-column). The water injections were made in splitless mode to maximize column exposure to water, creating a more severe test; the standard injection was made in split mode under the conditions described above that had previously been shown to provide consistently good chromatographic performance for glycols. Peak symmetry was evaluated after every 10 water injections and the procedure was repeated until either peak symmetry fell below 0.50 or the column had reached 600 water injections.
The standard used for lifetime testing was a mixed glycol standard (cat.# 30471) containing propylene glycol and ethylene glycol diluted down to 50 µg/mL (ppm) in methanol:water (10:90). Preparing standards and samples in 10% methanol is recommended for routine analysis because it helps prevent the syringe needle from seizing up. 2-Butoxy ethanol was added as an internal standard at a final concentration of 500 ppm in each solution (10 ppm on-column).
Results and Discussion
Split injection was used here for the analysis of glycols in water because it provides rapid sample transfer and a narrow analyte band, which improves peak shape and retention time consistency. In addition, it reduces the amount of water in the injection port, which minimizes the potential for backflash. The 50:1 split injection used in this method effectively prevented sample expansion volume issues. Even though the amount of sample on column was significantly reduced, adequate sensitivity and linearity were still achieved for both analytes on an Rtx-Wax column, even after 600 water injections (Figures 1 and 2). In contrast, for the Stabilwax column, peak symmetry for propylene glycol fell below 0.5 after 350 injections, so linearity could not be reliably achieved after 600 injections.
Figure 1: Linear responses were obtained for propylene glycol (0.5–100 ng on-column) analyzed on an Rtx-Wax column even after 600 water injections.
Figure 2: Linear responses were obtained for ethylene glycol (0.5–100 ng on-column) analyzed on an Rtx-Wax column even after 600 water injections.
Column bleed is always a potential concern when analyzing low concentration compounds at high temperatures, as is the case with glycols analysis. Propylene glycol and ethylene glycol both have relatively high boiling points, 188 °C and 197 °C respectively, which require GC oven temperatures higher than 200 °C for proper elution. In addition, high molecular weight contaminants, which are frequently found in these environmental samples, may require temperatures of up to 240 °C for adequate removal. At these temperatures, column bleed can interfere with the ability to accurately analyze glycols at 10 ng on-column or below. Visual inspection of chromatographic baselines over the course of the lifetime experiment confirmed that both Rtx-Wax and Stabilwax columns showed very low bleed.
While both columns exhibited low bleed, the Rtx-Wax column had a much longer effective lifetime when challenged with repeated aqueous injections. The three Rtx-Wax columns tested all had excellent performance with no loss in peak shape or shift in retention time for either compound, even after 600 water injections (Figure 3). In fact, following the experiment, the final Rtx-Wax column tested remained in the instrument and still performed well even after 1600 water injections. In contrast, on the Stabilwax columns, peak symmetry began to deteriorate after 100 injections and was significantly worse after 350 injections (Figure 4), degrading to the point that column trimming was required. While Stabilwax columns have demonstrated stability and performance for many industrial chemicals and other applications, they are not as robust as Rtx-Wax columns when challenged with aqueous injections for the analysis of glycols. Rtx-Wax columns consistently provided excellent peak shape across multiple column lots and over the course of the entire study (Table I).
In addition to using the split injection method shown here with an Rtx-Wax column, labs analyzing glycols in water samples can further improve results by preventing carryover. Carryover is commonly associated with glycol analysis and can be caused by sample residue in the syringe being transferred from one injection to another. If the syringe is not properly cleaned between analyses or if 100% water is used as the rinse solvent, carryover will cause inconsistent results and may permanently damage the syringe plunger. Rinsing the syringe with methanol:water (50:50) three to six times between each injection will eliminate most sample residue and minimize the possibility of carryover.
Figure 3: Propylene glycol and ethylene glycol peak shapes and retention times on an Rtx-Wax column are virtually identical, even after the column was exposed to 600 water injections.
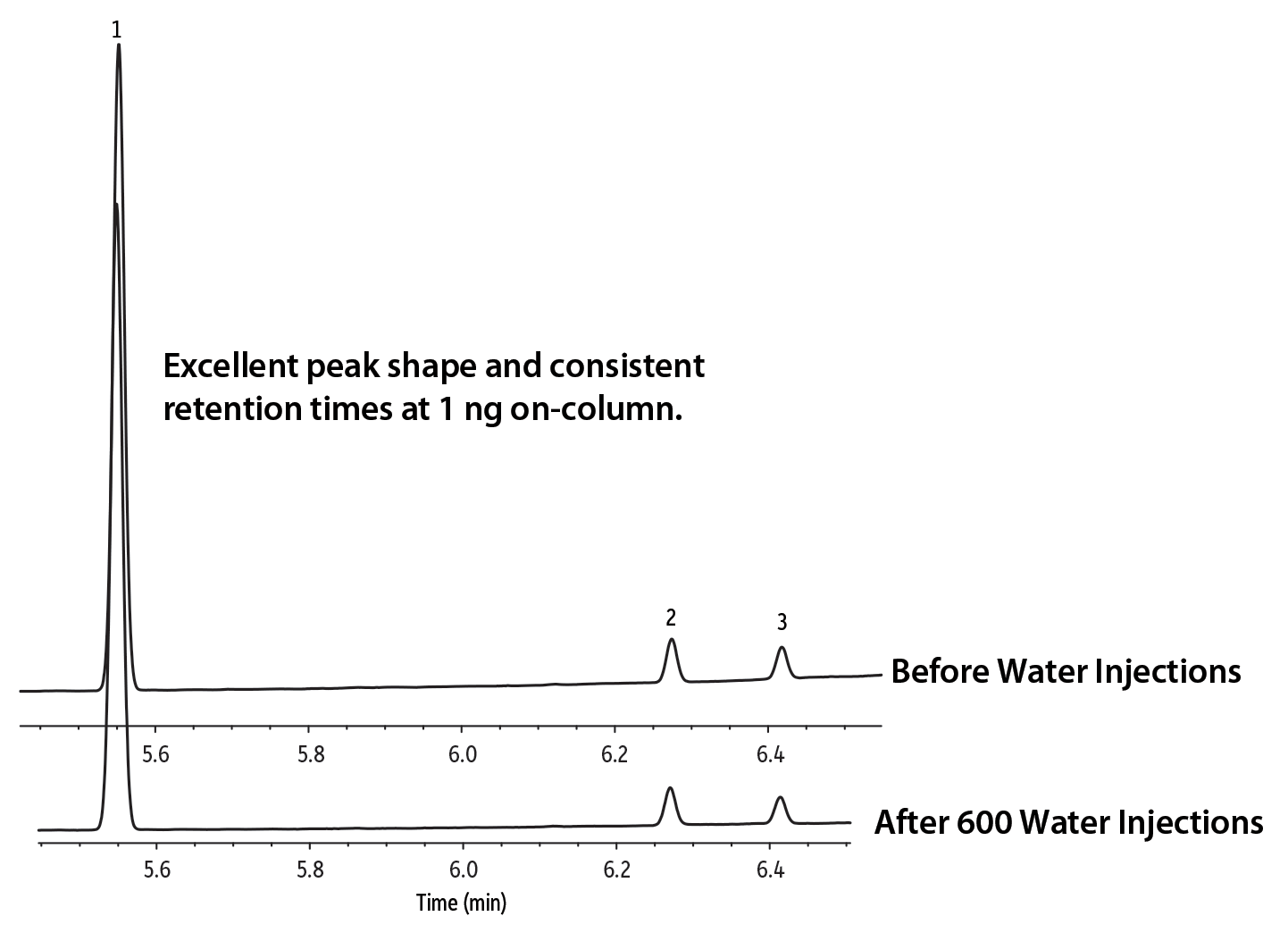
| Peaks | tR (min) | |
|---|---|---|
| 1. | 2-Butoxyethanol (IS) | 5.55 |
| 2. | Propylene glycol | 6.27 |
| 3. | Ethylene glycol | 6.41 |
| Column | Rtx-Wax, 30 m, 0.53 mm ID, 1.00 µm (cat.# 12455) |
|---|---|
| Standard/Sample | Glycols standard (cat.# 30471) |
| 2-Butoxyethanol | |
| Diluent: | Water:methanol (90:10) |
| Conc.: | 50 µg/mL (1 ng on-column) |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 50:1) |
| Liner: | Premium 4 mm Precision inlet liner w/wool (cat.# 23305) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 1 min) to 250 °C at 30 °C/min |
| Carrier Gas | He, constant flow |
| Flow Rate: | 5.7 mL/min |
| Linear Velocity: | 40 cm/sec |
| Detector | FID @ 250 °C |
|---|---|
| Make-up Gas Flow Rate: | 45 mL/min |
| Make-up Gas Type: | N2 |
| Hydrogen flow: | 40 mL/min |
| Air flow: | 450 mL/min |
| Data Rate: | 20 Hz |
| Instrument | Agilent/HP6890 GC |
Figure 4: Glycol peak symmetry declines significantly on a Stabilwax column after 350 injections of water.
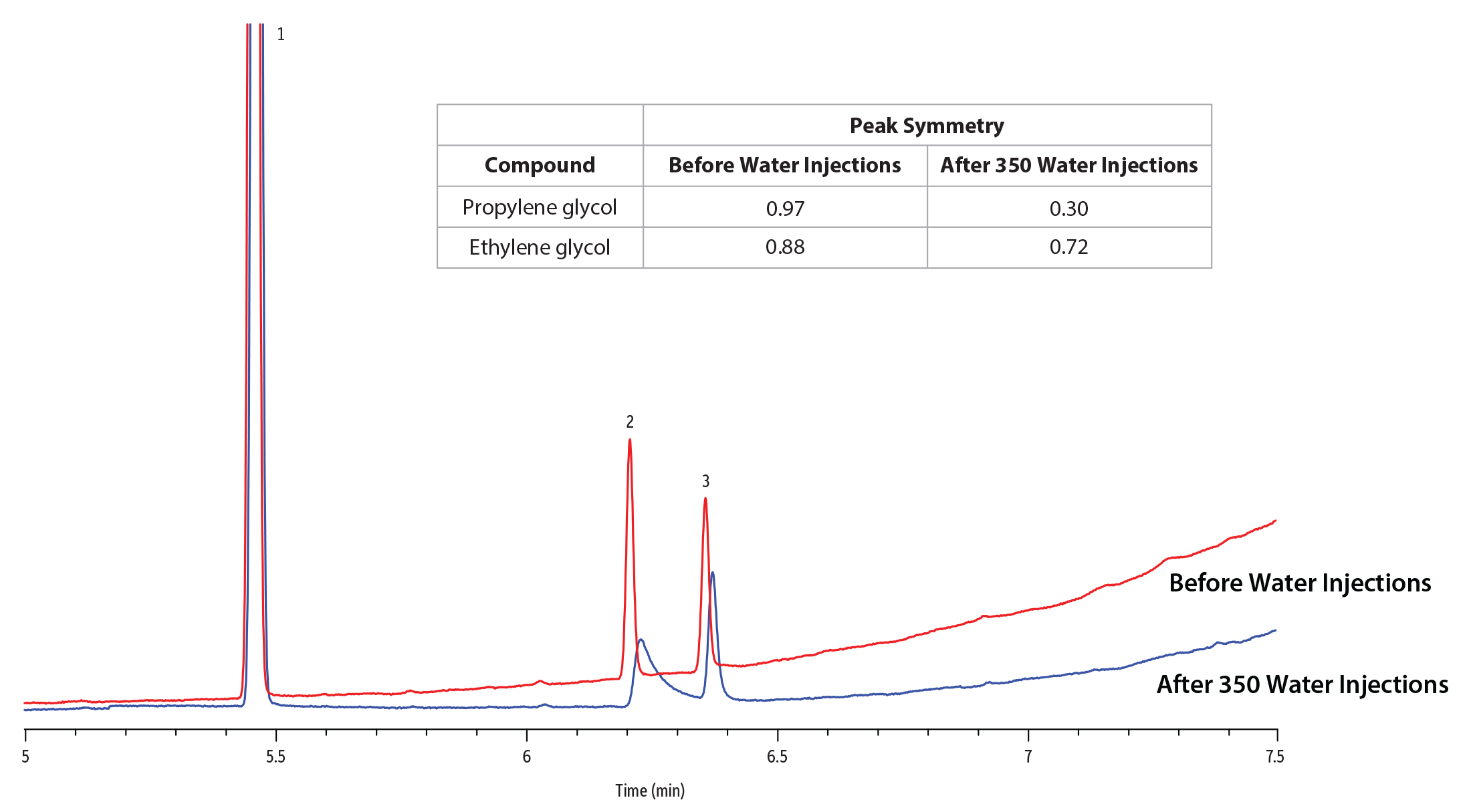
| Peaks | tR (min) | |
|---|---|---|
| 1. | 2-Butoxyethanol (IS) | 5.44 |
| 2. | Propylene glycol | 6.21 |
| 3. | Ethylene glycol | 6.36 |
| Column | Stabilwax, 30 m, 0.53 mm ID, 1.00 µm (cat.# 10655) |
|---|---|
| Standard/Sample | Glycols standard (cat.# 30471) |
| 2-Butoxyethanol | |
| Diluent: | Water:methanol (90:10) |
| Conc.: | 50 µg/mL (1 ng on-column) |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 50:1) |
| Liner: | Premium 4.0 mm Precision inlet liner w/wool (cat.# 23305) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 1 min) to 250 °C at 30 °C/min |
| Carrier Gas | He, constant linear velocity |
| Linear Velocity: | 40 cm/sec |
| Detector | FID @ 250 °C |
|---|---|
| Make-up Gas Flow Rate: | 45 mL/min |
| Make-up Gas Type: | N2 |
| Hydrogen flow: | 40 mL/min |
| Air flow: | 450 mL/min |
| Data Rate: | 20 Hz |
| Instrument | Agilent/HP6890 GC |
Table I: Rtx-Wax columns produced symmetrical peaks without column maintenance even after 600 water injections.
| Average Peak Symmetry at 1 ng On-Column (Rtx-Wax, n = 3) | |||
|---|---|---|---|
| # of Water Injections | IS | Propylene Glycol | Ethylene Glycol |
| 100 | 0.97 | 0.96 | 0.99 |
| 200 | 0.98 | 0.95 | 0.98 |
| 300 | 0.98 | 0.95 | 0.99 |
| 400 | 0.96 | 0.95 | 0.99 |
| 500 | 0.97 | 0.94 | 1.00 |
| 600 | 0.97 | 0.92 | 0.99 |
| %RSD | 0.5% | 1.2% | 0.7% |
Conclusion
Analyzing glycols in water samples using split injection provides several advantages over the typical splitless approach; it minimizes the risk of backflash and also prevents problems with peak tailing and shifting retention times. Using the split injection method demonstrated here with an Rtx-Wax column, very consistent chromatographic performance was obtained, even following the injection of 600 water samples. Because split injection on an Rtx-Wax column delivers fast, consistent sample transfer with less water on-column, this method produces symmetrical peaks that elute at stable retention times, which improves reproducibility for low-level analysis of glycols in water.
References
- J.D. Laukkonen, The history of antifreeze, Crankshift, March 1, 2017. http://www.crankshift.com/history-of-antifreeze/
- S. Ritter, What’s that stuff?, C&EN, January 1st, 2001. https://pubs.acs.org/cen/whatstuff/stuff/7901scit5.html
- FracFocus, Chemical disclosure registry, what chemicals are used. https://fracfocus.org/chemical-use/what-chemicals-are-used

