The Advantage of 2.1 mm ID Columns for LC-MS/MS Analysis of Drugs of Abuse
Introduction
The biphenyl phase lends superior selectivity over a C18 column for drugs of abuse (DOA) panels [1], but choosing the right column dimension for analysis is key for obtaining robust and accurate data. Each column dimension can be advantageous in different scenarios, but generally clinical labs are all working towards the same goals: high throughput, low sample volume, good sensitivity, and low cost. In this article, the advantages of narrow-bore columns will be discussed and demonstrated for drugs of abuse.
Efficiency
Column efficiency is the ability of a column to produce narrow chromatographic peaks and is defined by plate number (N) following Equation 1:
Equation 1:
Where Rt is the retention time of the analyte and W is peak width at baseline.
There can be several contributing factors for peak width and retention time that will ultimately determine the efficiency of the column. Band-broadening processes are factors that contribute to peak dispersion. These include Eddy diffusion, longitudinal mass transfer, and mobile phase and stationary phase mass transfer, which all collectively make up the van Deemter equation [2]. Eddy diffusion occurs when analytes take different flow paths through the column, which is exacerbated when using larger ID columns, longer column dimensions, larger particle sizes, and columns with poor packing efficiency. When a sample is injected on a column, the analyte is most concentrated in the middle, and as this band moves through the column, the analytes get dispersed outward. This is referred to as longitudinal mass transfer and can be reduced by running at higher flow rates. Mass transfer of the mobile phase and stationary phase is defined as analyte partitioning in and out of the stationary phase. This contributing factor to band broadening can be reduced by using smaller particle sizes and elevating the column oven temperature to increase the rate of diffusion. In general, using narrow-bore columns will result in less band broadening, increased peak efficiency, and improved analyte sensitivity. Particle type can also aid in increasing efficiency. Superficially porous particles decrease the analyte flow path and allow for increased efficiency when compared to fully porous particles.
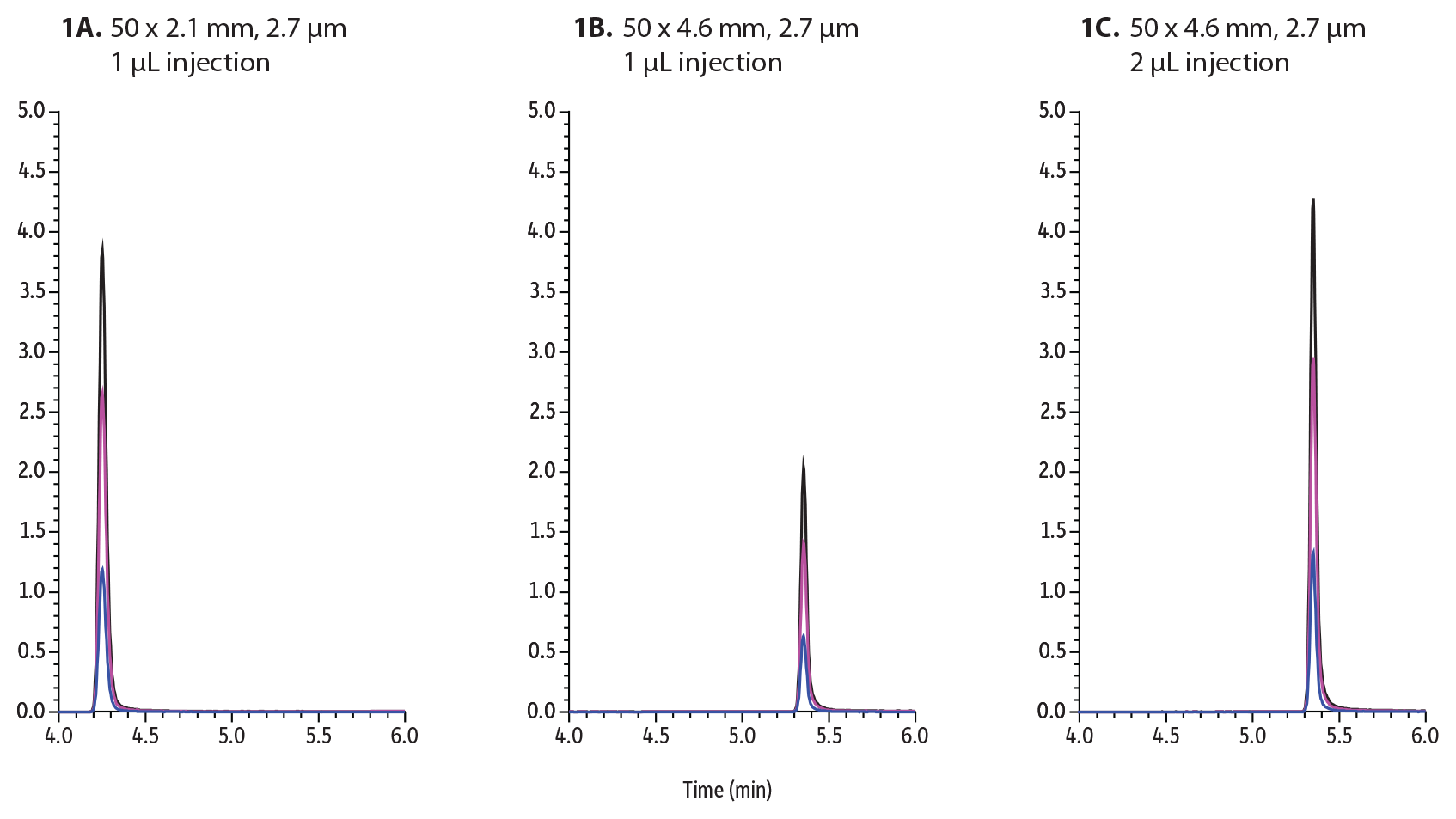
To demonstrate the superior sensitivity of narrow-bore columns, buprenorphine was investigated on Raptor Biphenyl 50 mm columns. In the following example (Figure 1A), 1 µL of 50 ppb buprenorphine was injected and analyzed by the outlined method using a 2.1 mm internal diameter column. Next, the same method was used on a 4.6 mm internal diameter column, adjusting the flow rate from 0.6 mL/min to 0.9 mL/min and injecting the same sample (Figure 1B). When the same amount of sample is injected on both columns, the larger-bore column produces approximately half of the peak height/sensitivity as the smaller-bore column. The sensitivity of analytes can be increased by injecting more on the larger ID column. In this example, when injecting twice as much onto the 4.6 mm ID column (2 µL), approximately the same sensitivity can be achieved (Figure 1C). The downside to increasing injection volume is the increased introduction of matrix on column that can adversely affect chromatography, decrease column lifetime, and enhance matrix interferences.
Figure 1: Buprenorphine was analyzed and injected onto these 50 mm Biphenyl columns:
A: 1 µL on 2.1 mm ID column
B: 1 µL on a 4.6 mm ID column
C: 2 µL on a 4.6 mm ID column
| Column | See notes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 45 °C | |||||||||||||||||||||
| Standard/Sample | ||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | |||||||||||||||||||||
| Conc.: | 50 ng/mL | |||||||||||||||||||||
| Mobile Phase | ||||||||||||||||||||||
| A: | Water, 0.1% formic acid | |||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | |||||||||||||||||||||
| ||||||||||||||||||||||
| Flow: | 0.6-0.9 mL/min |
| Detector | Shimadzu 8060 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Standards were aliquoted into 2 mL, screw-thread vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
| Notes | Figure 1A Column: Raptor Biphenyl 50 x 2.1 mm, 2.7 µm (cat.# 9309A52) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) Inj. Vol.: 1 µL Flow (mL/min): 0.6 Figure 1B Column: Raptor Biphenyl 50 x 4.6 mm, 2.7 µm (cat.# 9309A55) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 4.6 mm ID, 2.7 µm (cat.# 9309A0250) Inj. Vol.: 1 µL Flow (mL/min): 0.9 Figure 1C Column: Raptor Biphenyl 50 x 4.6 mm, 2.7 µm (cat.# 9309A55) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 4.6 mm ID, 2.7 µm (cat.# 9309A0250) Inj. Vol.: 2 µL Flow (mL/min): 0.9 |
Sensitivity can become even more challenging when applied to matrix. In the next example buprenorphine is spiked at 2 ng/mL into urine, hydrolyzed, diluted 30-fold, and centrifuged.
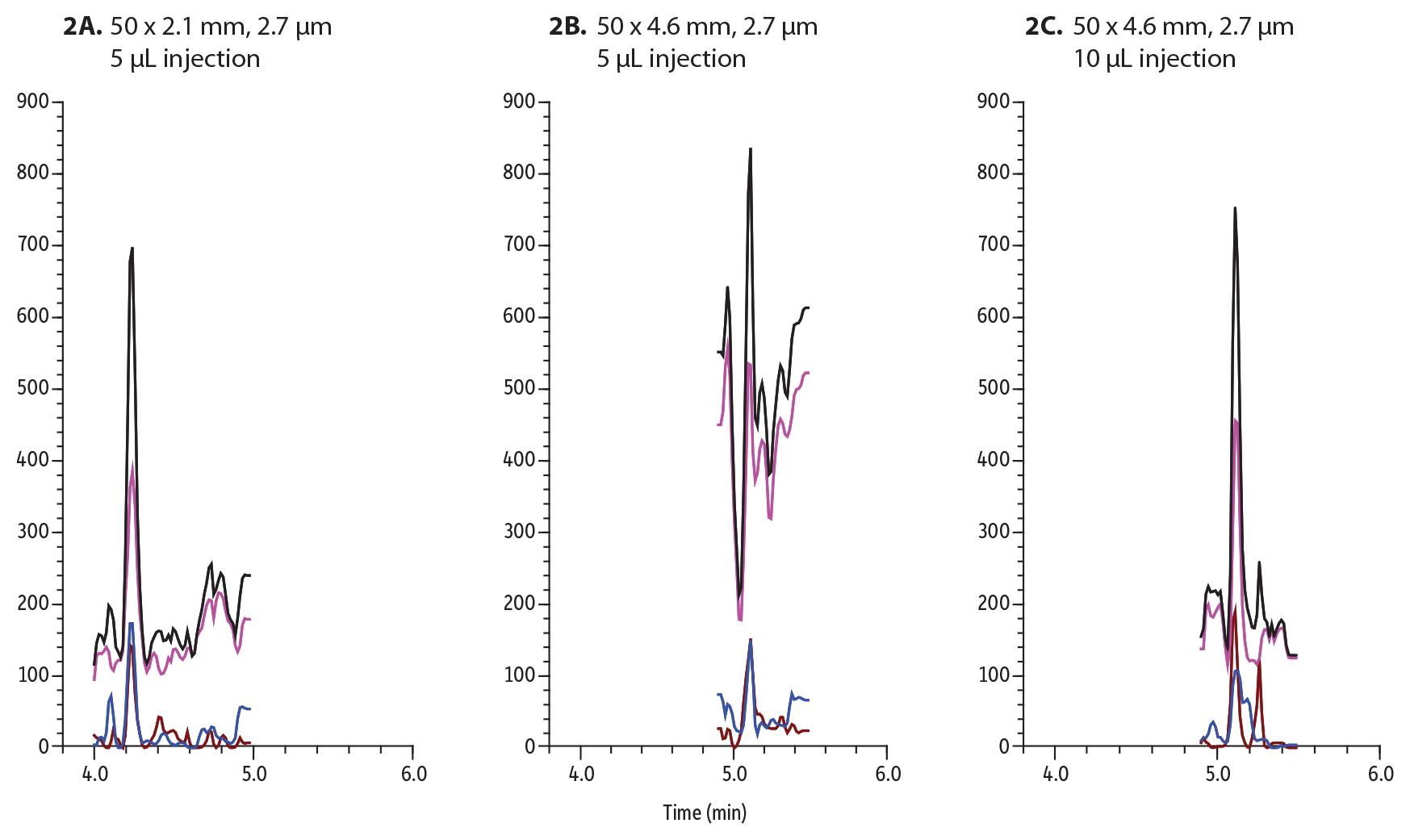
Figure 2: Buprenorphine was spiked into urine at 2 ng/mL and analyzed and injected onto these 50 mm Biphenyl columns:
A: 5 µL on 2.1 mm ID column
B: 5 µL on a 4.6 mm ID column
C: 10 µL on a 4.6 mm ID column
| Column | See notes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 45 °C | |||||||||||||||||||||
| Standard/Sample | ||||||||||||||||||||||
| Diluent: | 90:10 Water:methanol containing 0.1% formic acid | |||||||||||||||||||||
| Conc.: | 2 ng/mL in urine, diluted 30-fold | |||||||||||||||||||||
| Mobile Phase | ||||||||||||||||||||||
| A: | Water, 0.1% formic acid | |||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | |||||||||||||||||||||
| ||||||||||||||||||||||
| Flow: | 0.6-0.9 mL/min |
| Detector | Shimadzu 8060 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Buprenorphine was spiked at 2 ng/mL into urine, hydrolyzed, diluted 30-fold, and centrifuged. The sample was aliquoted into 2 mL, screw-threat vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
| Notes | Figure 2A Column: Raptor Biphenyl 50 x 2.1 mm, 2.7 µm (cat.# 9309A52) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) Inj. Vol.: 5 µL Flow (mL/min): 0.6 Figure 2B Column: Raptor Biphenyl 50 x 4.6 mm, 2.7 µm (cat.# 9309A55) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 4.6 mm ID, 2.7 µm (cat.# 9309A0250) Inj. Vol.: 5 µL Flow (mL/min): 0.9 Figure 2C Column: Raptor Biphenyl 50 x 4.6 mm, 2.7 µm (cat.# 9309A55) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 4.6 mm ID, 2.7 µm (cat.# 9309A0250) Inj. Vol.: 10 µL Flow (mL/min): 0.9 |
Figure 2 outlines the importance of sensitivity when analyzing matrix. The injection volume must be increased to 5 µL to be able to detect 2 ng/mL in urine on a 2.1 mm ID column. When analyzing the same amount (5 µL) on a 4.6 mm ID column, buprenorphine could not be detected. To achieve approximately the same amount of sensitivity, the injection volume had to be increased to 10 µL. The increase in sample volume also means an increase in matrix being injected onto column. To best conserve sample and maximize sensitivity, narrow-bore columns are favored.
To produce precise and rugged quantitative methods, a resolution of 1.5 or greater should be achieved. In the next example, nine groups of drugs of abuse isobars were analyzed on a Raptor Biphenyl 50 x 2.1 mm column and their resolutions calculated using Equation 2.
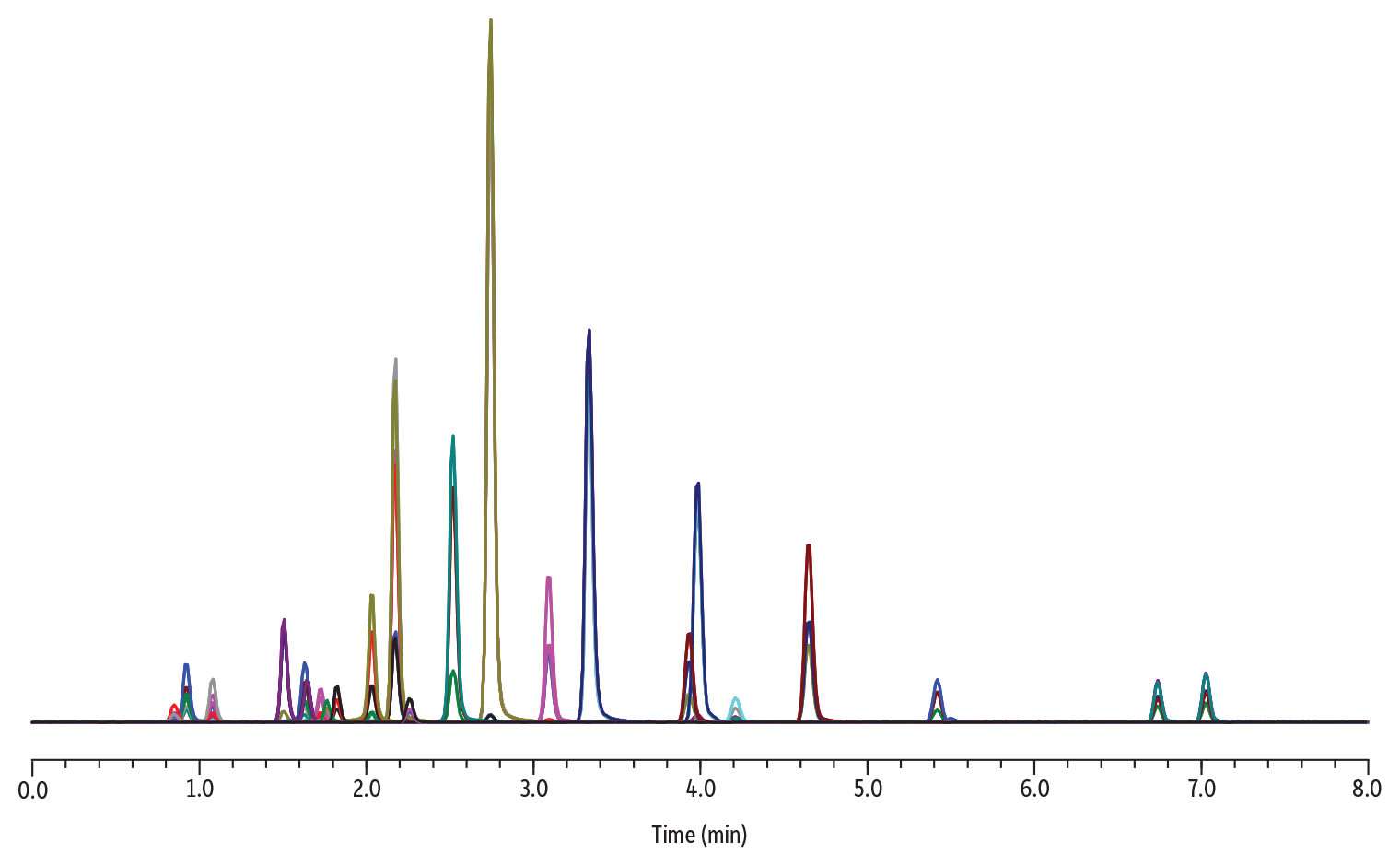
Figure 3: Chromatogram and Conditions for the Analysis of Isobar Panel
| Column | Raptor Biphenyl (cat.# 9309A52) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||||||||||
| Temp.: | 45 °C | ||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||||||||||||||||||
| Conc.: | 50 ng/mL | ||||||||||||||||||||||||||||
| Inj. Vol.: | 1 µL | ||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||||||||||
|
| Detector | Shimadzu 8060 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | The sample was aliquoted into 2 mL, screw-threat vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
Equation 2:
Where Rt is the retention time for the compounds that are being compared and W is the width of the peak at baseline.
Table I: Compound Name; Shared Molecular Weight; Analyte Retention Time (Min); Peak Width; and Calculated Resolution between Isobar Groups Using Equation 2
| Isobar Group | Name | Molecular Weight (g/mol) | Retention Time (min) | Peak Width | Resolution |
| 1 | Methamphetamine | 149.23 | 1.51 | 0.091 | 1.5 |
| Phentermine | 1.65 | 0.096 | |||
| 2 | Oxymorphone | 301.34 | 0.92 | 0.094 | 7.5 |
| Noroxycodone | 1.63 | 0.094 | |||
| 3 | Citalopram | 324.39 | 3.93 | 0.108 | 13.7 |
| Alpha-hydroxyalprazolam | 5.42 | 0.109 | |||
| 4 | Naloxone | 327.27 | 1.64 | 0.099 | 2.0 |
| 6-acetylmorphine | 1.82 | 0.082 | |||
| 5 | Morphine | 285.34 | 0.85 | 0.102 | 2.3 |
| Hydromorphone | 1.08 | 0.097 | |||
| Norhydrocodone | 1.73 | 0.085 | 7.1 | ||
| 7-aminoclonazepam | 3.09 | 0.099 | 14.9 | ||
| 6 | Lamotrigine | 256.09 | 2.26 | 0.093 | 2.7 |
| Hydroxybupropion | 255.74 | 2.52 | 0.095 | ||
| 7 | Codeine | 299.36 | 1.76 | 0.088 | 3.1 |
| Hydrocodone | 2.03 | 0.089 | |||
| 8 | O-desmethylvenlafaxine | 263.37 | 2.17 | 0.091 | 6.3 |
| Tramadol | 2.74 | 0.091 | |||
| Mirtazapine | 3.33 | 0.097 | 6.3 | ||
| Nortriptyline | 4.65 | 0.110 | 12.7 | ||
| 9 | CBD | 314.47 | 6.74 | 0.102 | 2.8 |
| 9-THC | 7.03 | 0.104 |
In this example, all pairs of isobars return a resolution value of 1.5 or greater and are retained well on a 2.1 mm ID column. Several contributing factors are at play to achieve acceptable resolution, including the superior selectivity of the biphenyl phase, the pairing to superficially porous particles, and the narrow-bore column mitigating band-broadening contributions.
The Green Advantage
The use of narrow-bore column dimensions oftentimes means greener solutions, which in this case means being more environmentally friendly while also reducing costs. Smaller ID columns can be advantageous over larger diameter columns when considering the consumption of solvent, as typically the larger the column internal diameter the higher the flow rate. Higher flow rates also hinder ionization efficiency for MS detection and can reduce sensitivity. In the following example, DOA isobars are compared using two different internal diameter columns, 2.1 and 4.6 mm.
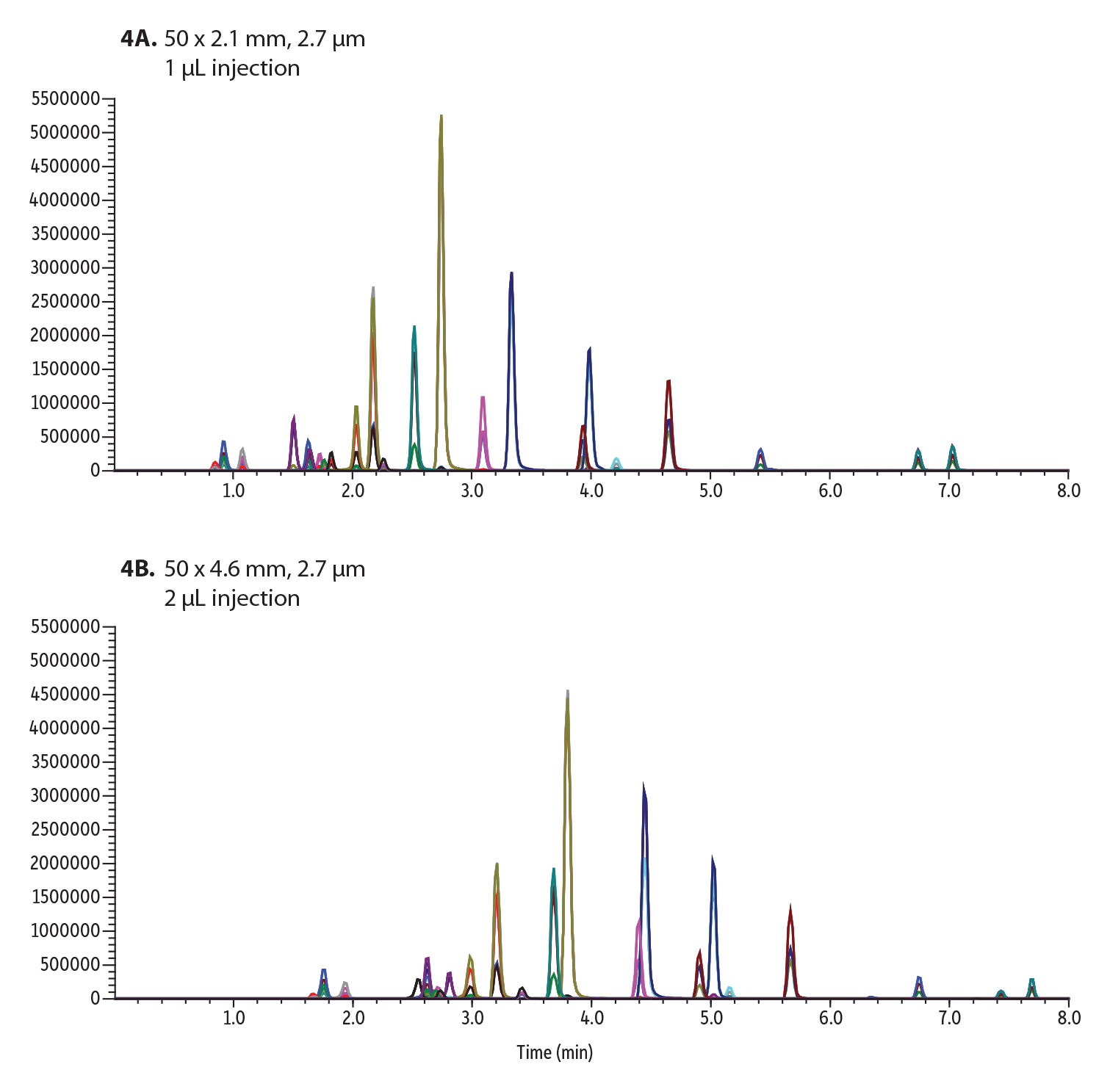
Figure 4: 22 Isobar Drugs of Abuse Compounds Analyzed on a Raptor Biphenyl Column Using:
A: 0.6 mL/min flow rate
B: 0.9 mL/min flow rate
| Column | See notes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp.: | 45 °C | |||||||||||||||||||||
| Standard/Sample | ||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol | |||||||||||||||||||||
| Conc.: | 50 ng/mL | |||||||||||||||||||||
| Mobile Phase | ||||||||||||||||||||||
| A: | Water, 0.1% formic acid | |||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | |||||||||||||||||||||
| ||||||||||||||||||||||
| Flow: | 0.6-0.9 mL/min |
| Detector | Shimadzu 8060 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Standards were aliquoted into 2 mL, screw-threat vials (cat.# 21143) and capped with short-cap, screw-vial closures (cat.# 24498). |
| Notes | Figure 4A Column: Raptor Biphenyl 50 x 2.1 mm, 2.7 µm (cat.# 9309A52) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) Inj. Vol.: 1 µL Flow (mL/min): 0.6 Figure 4B Column: Raptor Biphenyl 50 x 4.6 mm, 2.7 µm (cat.# 9309A55) Guard: Raptor Biphenyl EXP guard column cartridge 5 mm, 4.6 mm ID, 2.7 µm (cat.# 9309A0250) Inj. Vol.: 2 µL Flow (mL/min): 0.9 |
While the run time does not increase in this example, which can sometimes be the case when transferring to a larger-bore column, the flow rate did need to be adjusted from 0.6 mL/min to 0.9 mL/min. This means that the consumption of solvent has increased by one third per minute, costing more money than the smaller bore column, and generating more waste. Since acceptable resolution is already achieved on the 2.1 mm ID column, there is minimal advantage to moving to larger column IDs. In the long run, smaller columns will ultimately result in less solvent consumption and more cost-efficient solutions.
Robustness in matrix
To demonstrate the narrow-bore column’s robustness in matrix over time, 1000 injections were performed on a 2.1 mm ID column in urine matrix. The goal of these experiments was to demonstrate column robustness in matrix showing four different metrics: sensitivity, instrument pressure, resolution of a critical pair, and performance of an early-eluting compound. To demonstrate sensitivity, buprenorphine was spiked into urine at 2 ng/mL and diluted 30-fold.
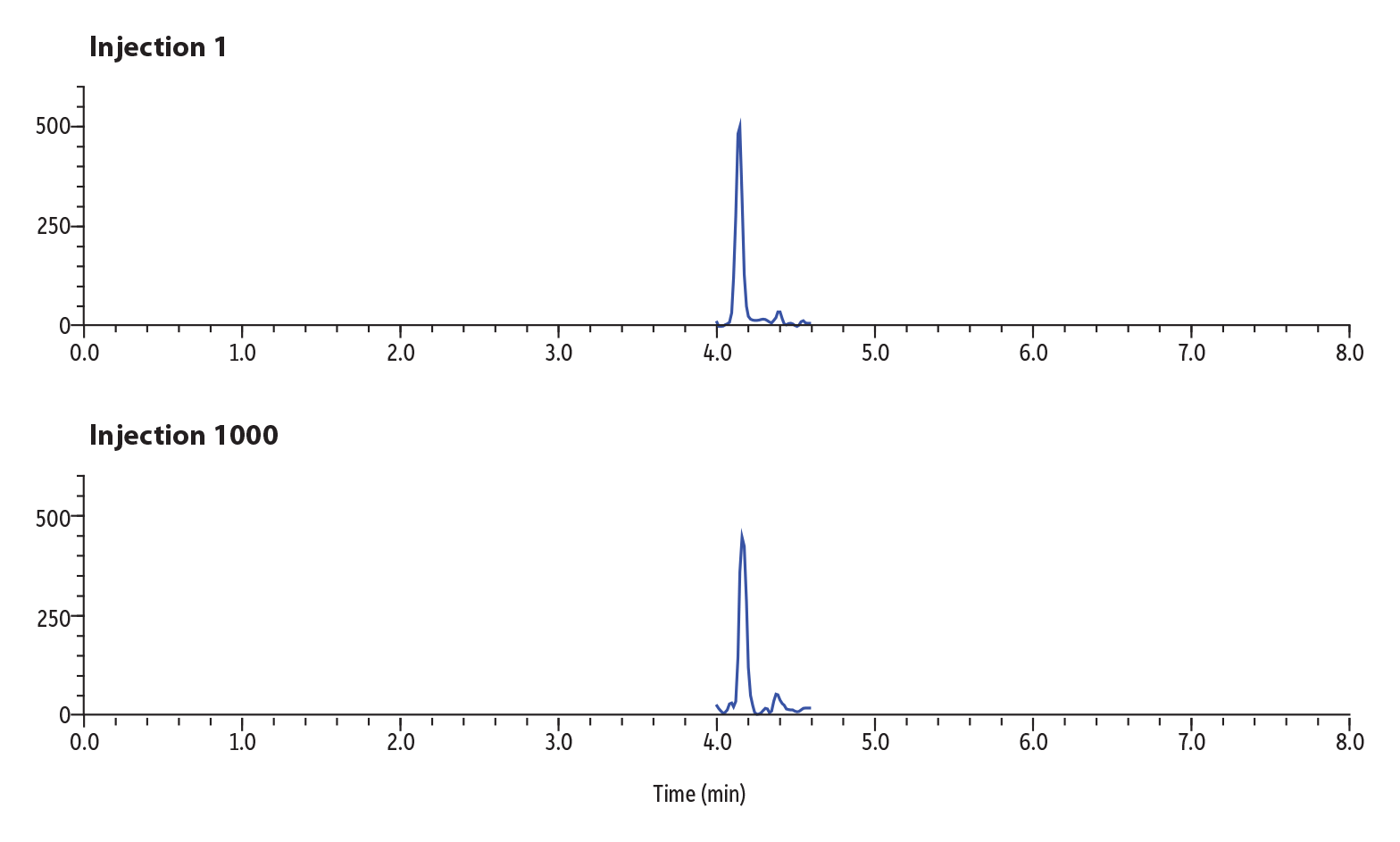
Figure 5: Lifetime Sensitivity Study of 1000 Injections of Buprenorphine in Urine.
Figure 5 shows the results of the sensitivity study over lifetime for buprenorphine. Buprenorphine was spiked into urine at 2 ng/mL and diluted in solvent 30-fold. Injection 1 in the lifetime study returned 1464 counts for peak area and 487 counts for peak height. Injection 1000 returned 1402 counts for peak area and 430 counts for height. The percent difference over the lifetime of the column was calculated to be 4% for peak area between injection 1 and 1000, demonstrating that sensitivity of the analyte has not deteriorated over time.
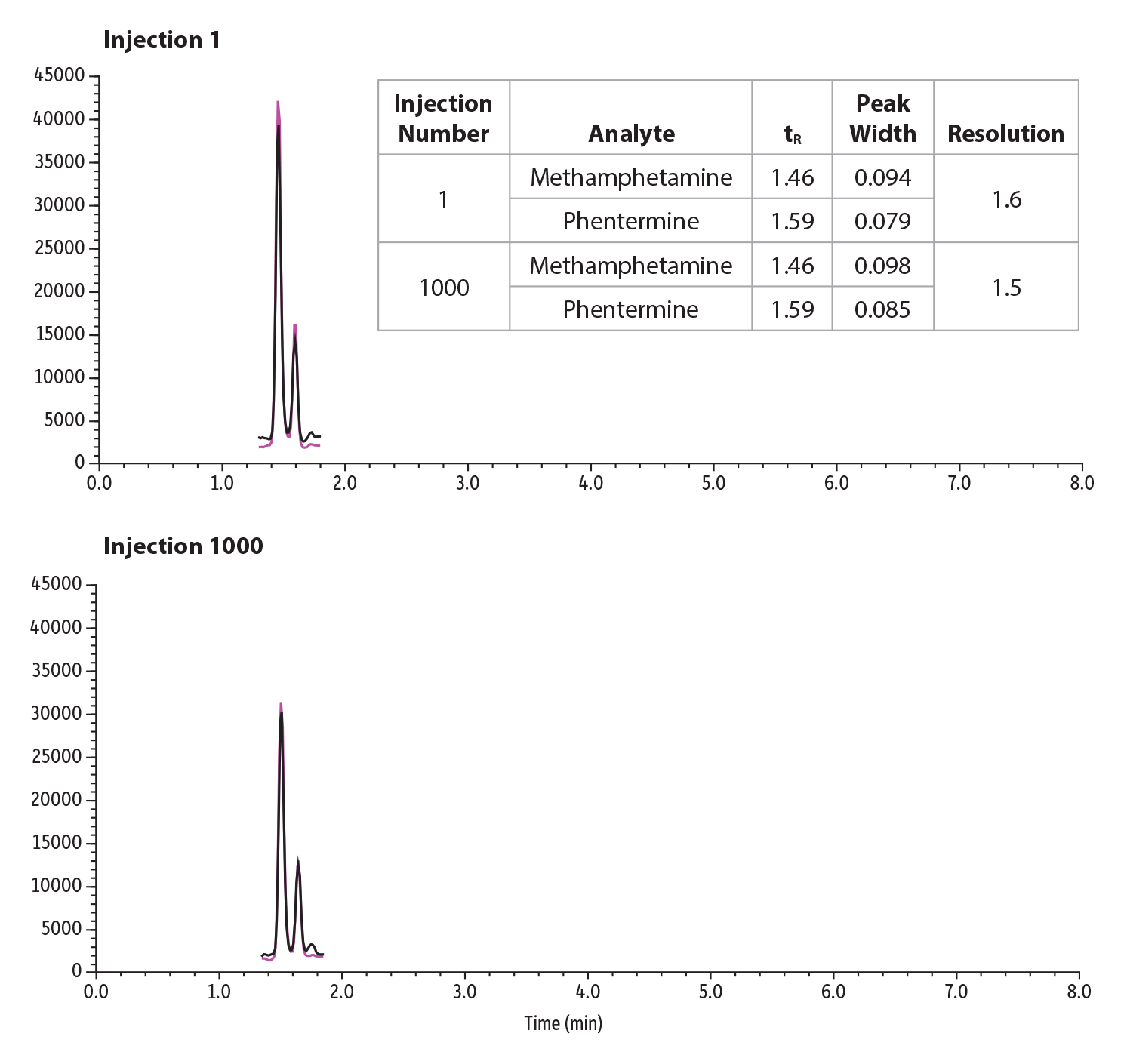
To demonstrate resolution stability over a 1000 injection lifetime study, phentermine and methamphetamine were spiked into urine at 2 ng/mL and diluted 30-fold. The retention time and peak width were extracted for injection 1 and 1000 and their resolution calculated. Resolution between the critical pairs was able to be maintained over the lifetime study and can be seen in Figure 6.
Figure 6: Lifetime Resolution Study of 1000 Injections of a Critical Pair, Phentermine and Methamphetamine, in Urine
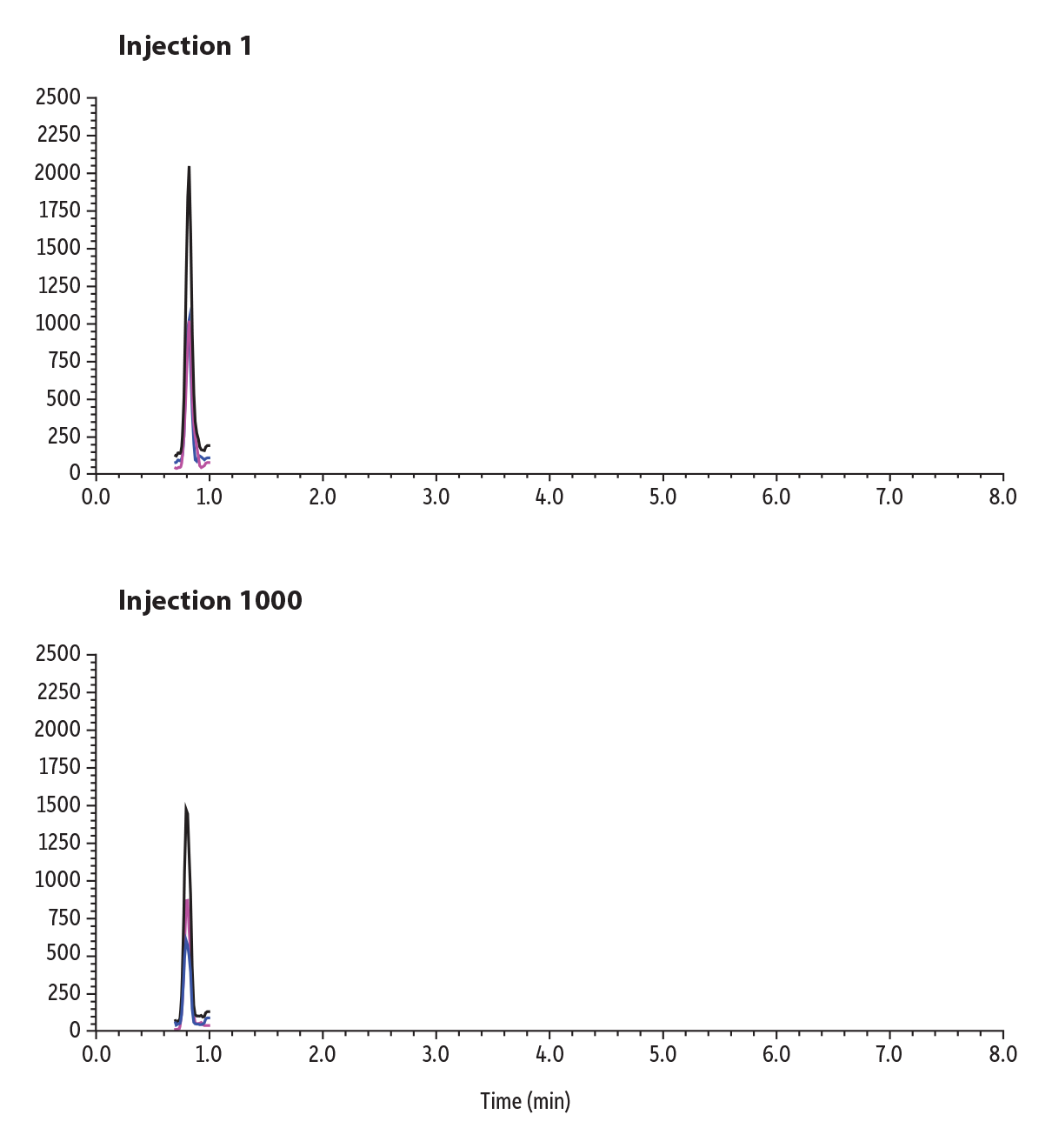
An early-eluting compound, morphine, was spiked into urine and shown over the lifetime study to demonstrate robustness in matrix and can be seen in Figure 7. While there is a decrease in sensitivity across 1000 injections, the column still demonstrates comparable retention to the first injection.
Figure 7: Lifetime Study of 1000 Injections of an Early-Eluting Compound, Morphine, in Urine
Finally, pressure profiles were collected from runs 1 and 1000 to compare. The pressure profiles were nearly identical, as seen in Figure 8, and demonstrate that debris is not being collected, and that the narrow-bore column is able to analyze matrix samples without increasing pressure or clogging.
Figure 8: Pressure Profiles for Runs 1 and 1000 in Urine Matrix on Raptor Biphenyl 50 x 2.1 mm, 2.7 µm Column
Conclusions
When choosing a column dimension for analysis, it’s important to keep in mind the benefits of narrow-bore columns. Small ID columns can be beneficial in the reduction of solvent and sample consumption for greener solutions and cost savings. The 2.1 mm ID columns demonstrate superior sensitivity over the 4.6 mm ID columns due to column efficiency, less analyte dilution, and reduced band broadening, while still achieving acceptable resolution for critical pairs. Even in matrix, the narrow-bore columns are reproducible and robust after repeat injections without increasing backpressure or clogging.
References
- S. Lupo, The big pain: Development of pain-free methods for analyzing 231 multiclass drugs and metabolites by LC-MS/MS, Application note, CFAR2309-UNV, Restek Corporation, 2015. https://www.restek.com/articles/the-big-pain-development-of-pain-free-methods-for-analyzing-231-multiclass-drugs-and-metabolites-by-lc-msms
- S. Lupo, LC-MS sensitivity: Practical strategies to boost your signal and lower your noise, LC GC N. Am., 36 (2018) (9) 652-660. https://www.chromatographyonline.com/view/lc-ms-sensitivity-practical-strategies-boost-your-signal-and-lower-your-noise