Fast, 8-Minute Method for Direct Injection of PFAS in Non-Potable Water
Featured Application: PFAS in Non-Potable Water on Force C18
- Analyze EPA Method 8327 PFAS 3x faster than method conditions.
- Direct injection means no time-consuming sample preparation steps.
- Force C18 FPP column provides extra retention and improved peak shape for PFBA.
While methods for analyzing per- and polyfluoroalkyl substances (PFAS) in drinking water typically use SPE sample preparation (styrene divinylbenzene or weak anion exchange), non-potable waters are most often analyzed using direct injection. EPA Method 8327, for example, is a validated test method used to measure a group of 24 PFAS compounds in groundwater, surface water, and wastewater samples using LC-MS/MS. Direct injection can be advantageous because time-consuming sample preparation and its associated costs and variability can be avoided, but the typical 21-minute analysis time limits sample throughput. To provide a faster approach for direct injection of PFAS, we have established a chromatographic method for the comprehensive analysis of the 24 target and 19 surrogate PFAS in EPA 8327 with a total cycle time of just 8 minutes. This method also provides proper detection sensitivity (10 ppt before dilution) for all analytes.
Using a 1:1 dilution with methanol (0.1% acetic acid) and the conditions shown below, all compounds were successfully analyzed on a Force C18 (50 mm x 2.1 mm, 3 µm) fully porous particle (FPP) column. The high surface area and carbon load of the FPP column provides increased chromatographic retention compared to a superficially porous particle (SPP) column, which can be advantageous when PFBA is a target analyte. As an early eluting compound, PFBA can be difficult to chromatograph, especially if there is a mismatch between the sample diluent and the initial mobile phase composition. When this occurs, PFBA peak shape can be very poor, and fronting or peak splitting may be observed. An effective way to address this is to increase the retention time of PFBA by using a Force C18 column. As shown below, labs running direct injection of PFAS can obtain excellent resolution of EPA Method 8327 compounds in a third of the time using a Force C18 column. In addition, labs dealing with poor retention and peak shape for PFBA on a superficially porous particle (SPP) column will benefit from using a Force C18 column under the conditions established here.
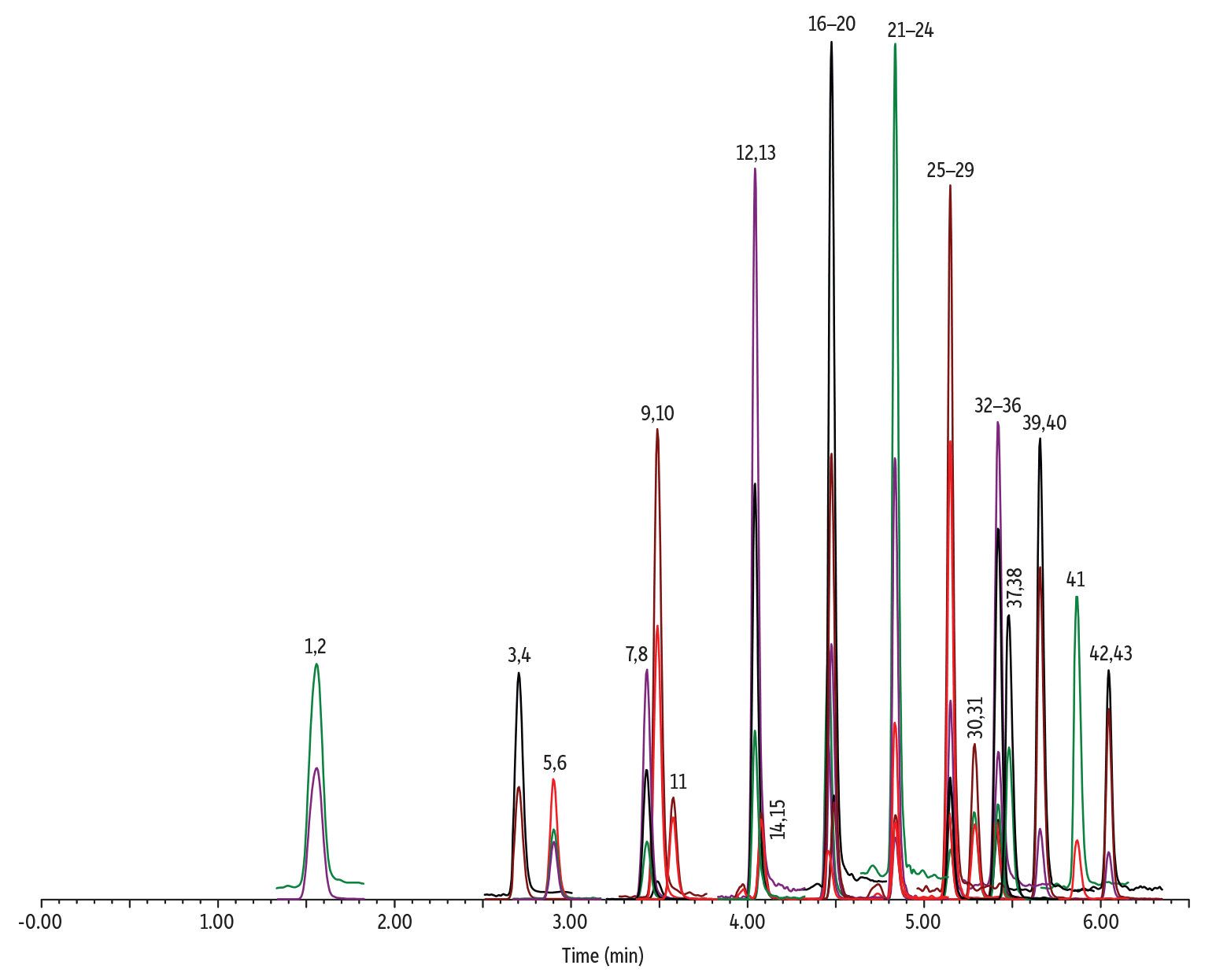
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Perfluorobutanoic acid (PFBA) | 1.56 | 80 | 231.1 | 169.1 | - |
| 2. | Perfluoro-n-[13C4]butanoic acid (M4PFBA) | 1.56 | 40 | 217.0 | 172.0 | - |
| 3. | Perfluoropentanoic acid (PFPeA) | 2.70 | 80 | 263.0 | 219.0 | - |
| 4. | Perfluoro-n-[13C5]pentanoic acid (M5PFPeA) | 2.70 | 40 | 268.0 | 223.0 | - |
| 5. | Perfluorobutane sulfonic acid (PFBS) | 2.90 | 80 | 299.1 | 80.0 | 99.0 |
| 6. | Perfluoro-1-[2,3,4-13C3]butyl sulfonic acid (M3PFBS) | 2.90 | 40 | 302.0 | 80.0 | - |
| 7. | 1H, 1H, 2H, 2H-perfluorohexane sulfonic acid (4:2 FTS) | 3.43 | 80 | 327.1 | 307.1 | 80.8 |
| 8. | 1H, 1H, 2H, 2H-perfluoro-1-[1,2-13C2]hexyl sulfonic acid (M2-4:2 FTS) | 3.43 | 40 | 329.0 | 309.0 | - |
| 9. | Perfluorohexanoic acid (PFHxA) | 3.49 | 80 | 313.1 | 269.1 | 119.1 |
| 10. | Perfluoro-n-[1,2,3,4,6-13C5]hexanoic acid (M5PFHxA) | 3.49 | 40 | 318.0 | 273.0 | - |
| 11. | Perfluoropentane sulfonic acid (PFPeS) | 3.58 | 80 | 349.1 | 80.0 | 99.0 |
| 12. | Perfluoroheptanoic acid (PFHpA) | 4.04 | 80 | 363.2 | 319.1 | 169.1 |
| 13. | Perfluoro-n-[1,2,3,4-13C4]heptanoic acid (M4PFHpA) | 4.04 | 40 | 367.0 | 322.0 | - |
| 14. | Perfluorohexane sulfonic acid (PFHxS) | 4.07 | 80 | 399.1 | 80.0 | 99.0 |
| 15. | Perfluoro-1-[1,2,3-13C3]hexyl sulfonic acid (M3PFHxS) | 4.07 | 40 | 402.0 | 80.0 | - |
| 16. | 1H, 1H, 2H, 2H-perfluorooctane sulfonic acid (6:2 FTS) | 4.46 | 80 | 427.2 | 407.2 | 80.7 |
| 17. | 1H, 1H, 2H, 2H-perfluoro-1-[1,2-13C2]octyl sulfonic acid (M2-6:2 FTS) | 4.46 | 40 | 429.0 | 409.0 | - |
| 18. | Perfluorooctanoic acid (PFOA) | 4.48 | 80 | 412.9 | 369.0 | 169.0 |
| 19. | Perfluoro-n-[13C8]octanoic acid (M8PFOA) | 4.48 | 40 | 421.0 | 376.0 | - |
| 20. | Perfluoroheptane sulfonic acid (PFHpS) | 4.49 | 80 | 449.2 | 80.0 | 99.0 |
| 21. | Perfluorononanoic acid (PFNA) | 4.84 | 80 | 463.2 | 419.2 | 219.1 |
| 22. | Perfluoro-n-[13C9]nonanoic acid (M9PFNA) | 4.84 | 40 | 472.0 | 427.0 | - |
| 23. | Perfluorooctane sulfonic acid (PFOS) | 4.84 | 80 | 499.2 | 80.0 | 99.0 |
| 24. | Perfluoro-1-[13C8]octyl sulfonic acid (M8PFOS) | 4.84 | 40 | 506.8 | 80.0 | - |
| 25. | Perfluorononane sulfonic acid (PFNS) | 5.14 | 80 | 549.2 | 80.0 | 99.0 |
| 26. | 1H, 1H, 2H, 2H-perfluorodecane sulfonic acid (8:2 FTS) | 5.15 | 80 | 527.2 | 507.2 | 80.8 |
| 27. | 1H, 1H, 2H, 2H-perfluoro-1-[1,2-13C2]decyl sulfonic acid (M2-8:2 FTS) | 5.15 | 40 | 529.0 | 509.0 | - |
| 28. | Perfluorodecanoic acid (PFDA) | 5.15 | 80 | 513.2 | 469.2 | 219.1 |
| 29. | Perfluoro-n-[1,2,3,4,5,6-13C6]decanoic acid (M6PFDA) | 5.15 | 40 | 518.9 | 473.9 | - |
| 30. | N-methylperfluoro-1-octanesulfonamidoacetic acid (N-MeFOSAA) | 5.28 | 80 | 570.2 | 419.2 | 483.2 |
| 31. | N-methyl-d3-perfluoro-1-octanesulfonamidoacetic acid (d3-N-MeFOSAA) | 5.28 | 40 | 572.9 | 418.9 | - |
| 32. | Perfluorodecane sulfonic acid (PFDS) | 5.40 | 80 | 599.2 | 80.0 | 99.0 |
| 33. | N-ethylperfluoro-1-octanesulfonamidoacetic acid (N-EtFOSAA) | 5.42 | 80 | 584.2 | 419.2 | 483.1 |
| 34. | N-ethyl-d5-perfluoro-1-catanesulfonamidoacetic acid (d5-N-EtFOSAA) | 5.42 | 40 | 589.0 | 419.0 | - |
| 35. | Perfluoroundecanoic acid (PFUnA) | 5.42 | 80 | 563.2 | 519.2 | 269.1 |
| 36. | Perfluoro-n-[1,2,3,4,5,6,7-13C7]undecanoic acid (M7PFUnA) | 5.42 | 40 | 569.9 | 524.9 | - |
| 37. | Perfluorooctane sulfonic acid (FOSA) | 5.49 | 80 | 498.2 | 78.0 | - |
| 38. | Perfluoro-1-[13C8]octanesulfonamide (M8FOSA) | 5.49 | 40 | 505.9 | 78.0 | - |
| 39. | Perfluorododecanoic acid (PFDoA) | 5.65 | 80 | 613.2 | 569.2 | 169.1 |
| 40. | Perfluoro-n-[1,2,-13C2]dodecanoic acid (M2PFDoA) | 5.65 | 40 | 614.8 | 569.9 | - |
| 41. | Perfluorotridecanoic acid (PFTriA) | 5.86 | 80 | 663.2 | 619.2 | 169.1 |
| 42. | Perfluorotetradecanoic acid (PFTreA) | 6.05 | 80 | 713.2 | 669.2 | 169.1 |
| 43. | Perfluoro-n-[1,2-13C2]tetradecanoic acid (M2PFTreA) | 6.05 | 40 | 714.8 | 669.7 | - |
| Column | Force C18 (cat.# 9634352) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 3 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | 50:50 Water:methanol, 0.1% acetic acid | ||||||||||||||||||||||||
| Conc.: | 80 ppt (40 ppt for surrogate or isotopically-labelled PFAS) | ||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |

