Fast Analysis of Fluorotelomer Alcohols
Featured Application: Fluorotelomer Alcohols (FTOHs) on LPGC Rtx-200
• 1.9x faster than conventional GC-MS analysis.
• Reduces helium usage by 60% compared to conventional GC-MS methods.
• LPGC kits are factory connected with a proven leak-free connector making LPGC as simple as a column change.
Fluorotelomer alcohols (FTOHs) have recently emerged as an important class of PFAS compounds in environmental testing laboratories. These compounds are commonly used in manufacturing materials for their oil and water-repellant capabilities but have recently been found to degrade into toxic compounds that pose health and environmental concerns. The degradation products of these volatile FTOHs are frequently detected in indoor and outdoor air samples and thus are important to monitor. Since FTOHs are an emerging class of compounds, testing methodologies are limited. In this application, we developed a rapid analysis of four common fluorotelomer alcohols on the LPGC Rtx-200, achieving excellent separations with a run time of under four minutes. As show in Figure 1, the optimized LPGC method is 1.9x faster and uses 60% less helium than conventional methods. Despite significant speeds gains, excellent separations were still achieved for all isobars.
Utilizing LPGC for analysis of FTOHs significantly reduces analysis time, improving lab productivity while also conserving helium. Each of our factory-coupled LPGC kits are individually tested, so you can have the assurance of a leak-free connection. To learn more about this powerful technique, visit www.restek.com/lpgc
Figure 1: Comparison of Conventional and LPGC-MS Analysis of Fluorotelomer Alcohols
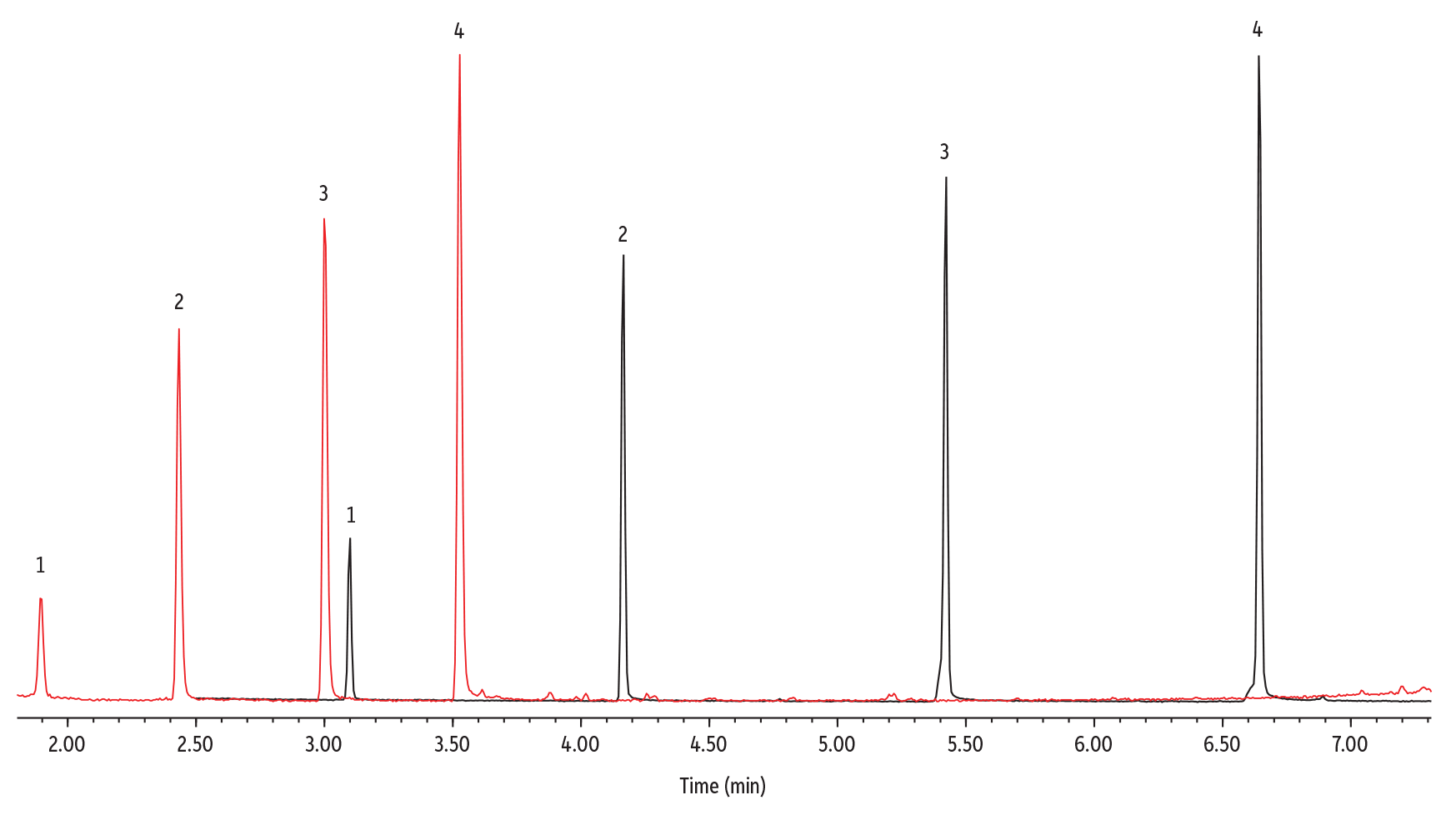
| Peaks | Conc. (µg/g) | tR (30 m) | tR (LPGC) | |
|---|---|---|---|---|
| 1. | 4:2 FTOH (2-perfluorobutyl alcohol) | 2 | 3.10 | 1.90 |
| 2. | 6:2 FTOH (2-perfluorohexyl alcohol) | 2 | 4.17 | 2.43 |
| 3. | 8:2 FTOH (2-perfluorooctyl alcohol) | 2 | 5.44 | 3.01 |
| 4. | 10:2 FTOH (2-perfluorodecyl alcohol) | 2 | 6.65 | 3.53 |
| Column | See notes |
|---|---|
| Standard/Sample | 2-(Perfluorobutyl)ethanol |
| 2-(Perfluorohexyl)ethanol | |
| 2-(Perfluorooctyl)ethanol | |
| 2-(Perfluorodecyl)ethanol | |
| Diluent: | Methanol |
| Conc.: | 2 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 5:1) |
| Liner: | Topaz, precision inlet liner, 4.0 mm x 6.3 x 78.5 (cat.# 23305) |
| Inj. Temp.: | 280 °C |
| Carrier Gas | He |
| Detector | MS | |
|---|---|---|
| Mode: | SIM | |
| SIM Program: | 131 m/z, 300 ms dwell | |
| Transfer Line Temp.: | 280 °C | |
| Analyzer Type: | Quadrupole | |
| Source Temp.: | 250 °C | |
| Quad Temp.: | 180 °C | |
| Tune Type: | PFTBA | |
| Ionization Mode: | EI | |
| Instrument | Agilent 7890B GC & 5977A MSD | |
| Sample Preparation | All standards (original concentration of 100 µg/mL) were combined into one solution at a concentration 1 ppm in polypropylene vial (cat. #23242) with a polypropylene cap (cat. #23244). A 50 µL aliquot was analyzed by GC-MS using a 100 µL insert (cat. #24512). | |
| Notes | Conventional (30 m) Analysis: Column: Rtx-200ms, 30 m, 0.25 mm ID, 0.25 µm (cat.# 15623) Temp. program: 35 °C (hold 1 min) to 280 °C at 15 °C/min (hold 5 min) Flow: 1.2 mL/min SIM start: 2.5 min SIM: 131 m/z, 300 ms LPGC-MS Analysis: Column: LPGC Rtx-200 column kit, includes 10 m x 0.32 mm ID x 1.00 µm Rtx-200 analytical column and 5 m x 0.15 mm ID Rxi restrictor factory connected via SilTite connector (cat.# 11807) Temp. program: 35 °C (hold 0.5 min) to 280 °C at 35 °C/min (hold 5 min) Flow: 0.9 mL/min SIM start: 1.5 min SIM: 131 m/z, 300 ms Pulsed split injection was used; 30 psi until 0.15 min. The injections were performed on different instruments under different head pressures, resulting in different analyte responses. | |

