Analyze EPA Method 533 PFAS Reliably with Resprep WAX SPE
Abstract
Low-level analysis of per-and polyfluoroalkyl substances (PFAS) requires high sensitivity and low background levels throughout the entire workflow from sample collection, transportation, and storage to analytical instrumentation and sample preparation. Resprep polymeric WAX SPE cartridges allow for accurate low-level quantitation of PFAS compounds in drinking water while meeting the requirements of EPA Method 533.
Introduction
Due to the low levels required by many regulatory agencies, the analysis of PFAS in drinking water often employs solid phase extraction (SPE) coupled with LC-MS/MS to reach low detection limits. EPA Method 533 [1] uses weak anion exchange (WAX) SPE to better retain short-chain acid compounds and obtain part-per-trillion (ppt) detection limits. However, due to the ubiquitous nature of PFAS compounds, the selection and screening of all materials and consumables that contact samples, including SPE cartridges, is critical for maintaining a clean background and allowing continued low-level analysis. In this assessment, Resprep WAX cartridges were used with a Resprep QR-12 vacuum manifold to extract samples for EPA Method 533 PFAS analysis using a Shimadzu Nexera LC and 8045 MS/MS.
Experimental
Sample Preparation
Spikes and blanks were prepared in polypropylene bottles using 250 mL deionized water spiked with isotope dilution standards as per EPA Method 533, Section 7.16.1. The 6 mL SPE cartridges, which contained 500 mg of 30 µm WAX (cat.# 28291), were placed on a Resprep QR-12 vacuum manifold (cat.# 28298-VM) that was fitted with quick-replace liners (cat.# 28310-VM). Resprep sample delivery system lines (cat.# 26250) were used to transfer the samples to the SPE cartridges. While both the quick-replace liners and sample delivery lines contain PTFE, which can be a potential source of PFAS contamination, investigation of blanks taken using all lines and all ports on the manifold showed no detectable PFAS leaching. Thorough and regular blank checking of all SPE components and solvents, especially when using new lots of materials, is recommended to ensure that background contamination is below acceptable levels.
After preparation of the samples and setup of the SPE system, the samples were extracted following the instructions in EPA Method 533, Section 11.4, which is summarized in Figure 1.
Figure 1: Sample Preparation Procedure for EPA Method 533 PFAS Analysis
Analytical System
After extraction, the samples were analyzed by LC-MS/MS under the EPA Method 533 PFAS analysis conditions shown below. The use of a PFAS delay column is important to prevent any PFAS contamination upstream of the injector from coeluting with the samples. Thorough blank checking of the analytical system was performed and showed no detectable PFAS contamination.
Instrument Conditions for EPA Method 533 PFAS Analysis
System: Shimadzu Nexera X2/Shimadzu LCMS-8045
Columns:
Injection volume: 3 µL
Mobile phase A: Water, 5 mM ammonium acetate
Mobile phase B: Methanol
Flow rate: 0.4 mL/min
Temperature: 40 °C
Gradient:
| Time (min) | %B |
| 0 | 20 |
| 6 | 95 |
| 6.6 | 95 |
| 6.61 | 20 |
| 7.5 | 20 |
Ion source: electrospray
Ion mode: ESI-
Mode: MRM
Method Detection Limits (MDL)
The method detection limit was calculated from the analysis of seven blank replicates and seven low-level spikes, as outlined in EPA’s Definition and Procedure for the Determination of the Method Detection Limit, Revision 2 [2]. The spikes were made at 0.5 ng/L using a 2 µg/mL stock solution of native PFAS compounds (EPA 533 PFAS calibration standard, cat.# 30736). The standard deviations of the spike and blank results were multiplied by the Student’s t-value of 3.143 to determine the MDL, and the higher of the results between the spikes and blanks was selected as the MDL.
Accuracy and Precision
Accuracy and precision were determined by analyzing five replicate 10 ng/L spikes. The accuracy of the spikes was calculated and compared to the recovery limits of 70-130% from EPA Method 533. The relative standard deviations of the spike results were also determined.
The recovery of the isotope dilution standards was calculated from the spike replicates and compared to the recovery limits of 50-200% from EPA Method 533 for PFAS analysis.
Results and Discussion
Good chromatographic results were obtained for all compounds, as shown in Figure 2. The MDL, accuracy, and precision results for native PFAS analytes are shown in Table I. The calculated MDLs were below the reporting limits shown in Table 7 in EPA Method 533, and the accuracy of the 10 ng/mL spikes ranged from 84 to 119% of the spiked value, well within the 70-130% recovery required by the method. The spikes showed good precision as well, with the results being ≤20% RSD.
Similarly, the recoveries for the isotope dilution standards were also within 20% of the true value and had precision ≤20% RSD. The results are shown in Table II.
Figure 2: Analysis of 25 ng/mL Standard for EPA Method 533
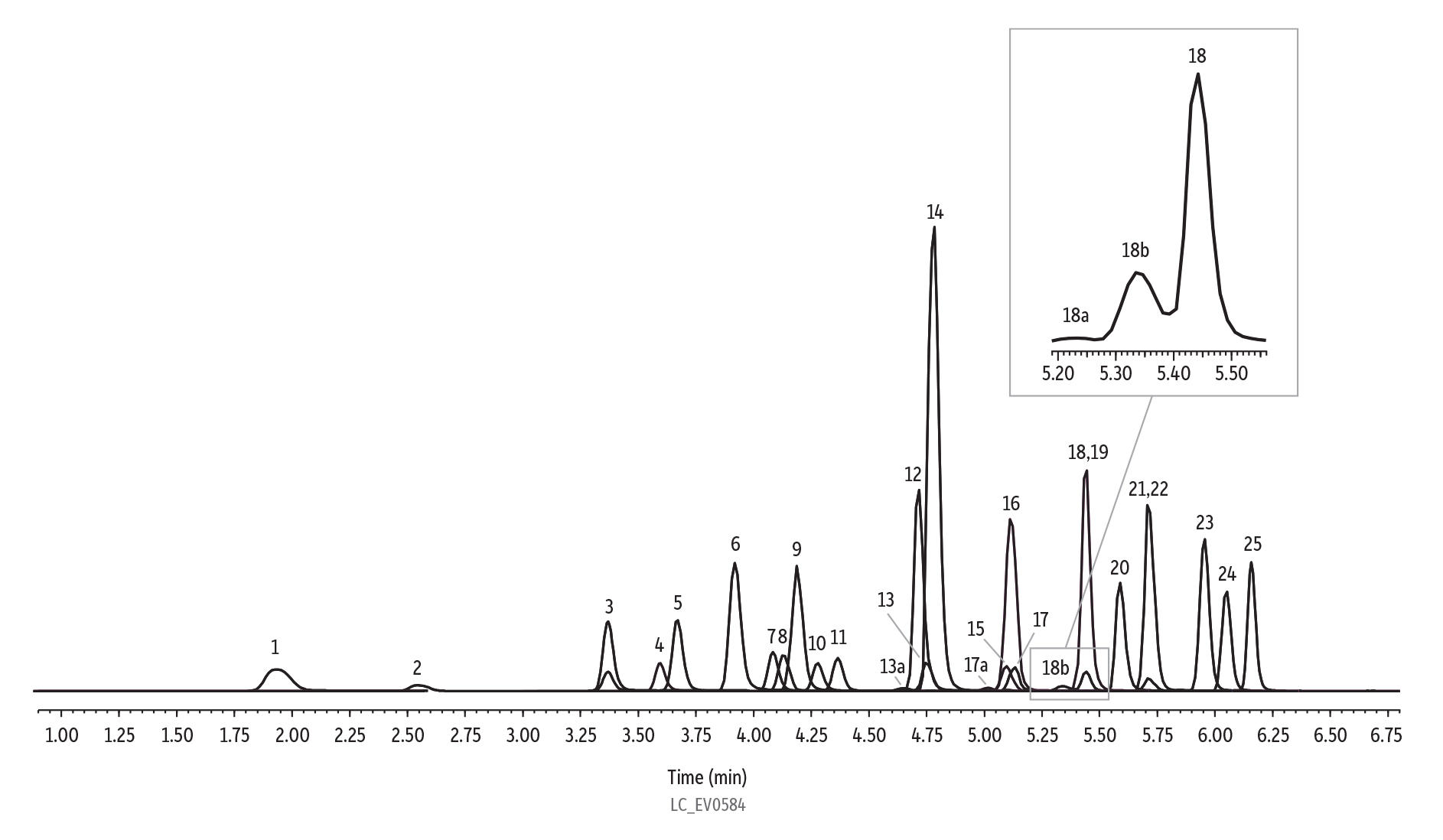
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | Perfluoro-n-butanoic acid (PFBA) | 1.933 | 25 | 213 | 169 |
| 2. | Perfluoro-3-methoxypropanoic acid (PFMPA) | 2.544 | 25 | 229 | 85 |
| 3. | Perfluoro-n-pentanoic acid (PFPeA) | 3.369 | 25 | 263 | 219 |
| 4. | Perfluorobutanesulfonic acid (PFBS) | 3.594 | 25 | 299 | 80 |
| 5. | Perfluoro-4-methoxybutanoic acid (PFMBA) | 3.669 | 25 | 279 | 85 |
| 6. | Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 3.919 | 25 | 315 | 135 |
| 7. | Perfluoro-3,6-dioxaheptanoic acid (NFDHA) | 4.084 | 25 | 295 | 201 |
| 8. | 1H,1H,2H,2H-Perfluorohexane sulfonic acid (4:2 FTS) | 4.129 | 25 | 327 | 307 |
| 9. | Perfluorohexanoic acid (PFHxA) | 4.189 | 25 | 313 | 269 |
| 10. | Perfluoro-1-pentanesulfonic acid (PFPeS) | 4.278 | 25 | 349 | 80 |
| 11. | Perfluoro(2-methyl-3-oxahexanoic) acid (HFPO-DA) | 4.365 | 25 | 285 | 169 |
| 12. | Perfluoroheptanoic acid (PFHpA) | 4.715 | 25 | 363 | 319 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 13. | Perfluoro-1-hexanesulfonic acid (PFHxS) | 4.750 | 25 | 399 | 80 |
| 14. | 4,8-dioxa-3H-perfluorononanoic acid (ADONA) | 4.78 | 25 | 277 | 251 |
| 15. | 1H,1H,2H,2H-Perfluorooctane sulfonic acid (6:2 FTS) | 5.096 | 25 | 427 | 407 |
| 16. | Perfluoro-1-heptanesulfonic acid (PFHpS) | 5.132 | 25 | 449 | 80 |
| 17. | Perfluorooctanoic acid (PFOA) | 5.115 | 25 | 413 | 369 |
| 18. | Perfluorooctanesulfonic acid (PFOS) | 5.441 | 25 | 499 | 80 |
| 19. | Perfluorononanoic acid (PFNA) | 5.439 | 25 | 463 | 419 |
| 20. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS) | 5.588 | 25 | 531 | 351 |
| 21. | 1H,1H,2H,2H-Perfluorodecane sulfonic acid (8:2 FTS) | 5.712 | 25 | 527 | 507 |
| 22. | Perfluorodecanoic acid (PFDA) | 5.712 | 25 | 513 | 469 |
| 23. | Perfluoroundecanoic acid (PFUnA) | 5.954 | 25 | 563 | 519 |
| 24. | 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 6.049 | 25 | 631 | 451 |
| 25. | Perfluorododecanoic acid (PFDoA) | 6.158 | 25 | 613 | 569 |
| Column | Force C18 (cat.# 9634252) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 1.8 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | EPA 533 PFAS calibration standard (cat.# 30736) | ||||||||||||||||||||||||
| Diluent: | 80:20 Methanol:water | ||||||||||||||||||||||||
| Conc.: | 25 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 3 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||
|
| Detector | Shimadzu LCMS-8045 |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Notes | Branched isomers for PFOA, PFOS, and PFHxS labeled as peak number "a" and "b." PFAS delay column used (cat.# 27854). |
Table I: Results from MDL, Precision, and Accuracy Experiments for Native PFAS
| Compound | Abbreviation | MDL (ng/L) | Accuracy (%) | %RSD |
| Perfluorobutanoic acid | PFBA | 8.5 | 95 | 20 |
| Perfluoro-3-methoxypropanoic acid | PFMPA | 0.2 | 119 | 20 |
| Perfluoropentanoic acid | PFPeA | 0.2 | 114 | 10 |
| Perfluorobutane sulfonate | PFBS | 0.3 | 94 | 14 |
| Perfluoro-4-methoxybutanoic acid | PFMBA | 1.1 | 88 | 8 |
| Perfluoro(2-ethoxyethane)sulfonic acid | PFEESA | 0.2 | 84 | 12 |
| Nonafluoro-3,6-dioxaheptanoic acid | NFDHA | 0.2 | 103 | 15 |
| 1H, 1H, 2H,2H-perfluorohexane sulfonate | 4:2 FTS | 0.3 | 97 | 19 |
| Perfluorohexanoic acid | PFHxA | 0.1 | 98 | 11 |
| Perfluoropentane sulfonate | PFPeS | 0.2 | 96 | 13 |
| Hexafluoropropylene oxide dimer acid | HFPO-DA | 1 | 89 | 13 |
| Perfluoroheptanoic acid | PFHpA | 0.4 | 101 | 17 |
| Perfluorohexane sulfonate | PFHxS | 0.3 | 110 | 15 |
| 4,8-Dioxa-3H-perfluorononanoic acid | ADONA | 1.1 | 100 | 7 |
| 1H, 1H, 2H,2H-perfluorooctane sulfonate | 6:2 FTS | 0.7 | 105 | 8 |
| Perfluoroheptane sulfonate | PFHpS | 0.5 | 101 | 12 |
| Perfluorooctanoic acid | PFOA | 0.2 | 112 | 9 |
| Perfluorooctane sulfonate | PFOS | 0.3 | 109 | 5 |
| Perfluorononanoic acid | PFNA | 0.5 | 101 | 10 |
| 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid | 9Cl-PF3ONS | 0.2 | 93 | 7 |
| Perfluorodecanoic acid | PFDA | 0.4 | 111 | 6 |
| 1H, 1H, 2H,2H-perfluorodecane sulfonate | 8:2 FTS | 0.6 | 109 | 5 |
| Perfluoroundecanoic acid | PFUnA | 0.8 | 110 | 6 |
| 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid | 11Cl-PF3OUdS | 0.3 | 95 | 11 |
| Perfluorododecanoic acid | PFDoA | 1.1 | 94 | 7 |
Table II: Results from Precision and Accuracy Experiments for Isotope Dilution Standards
| Compound | Abbreviation | Accuracy (%) | %RSD |
| Perfluoro-n-[1,2,3,4-13C4]butanoic acid | 13C4-PFBA | 111 | 13 |
| Perfluoro-n-[1,2,3,4,5-13C5]pentanoic acid | 13C5-PFPeA | 118 | 14 |
| Sodium perfluoro-1-[2,3,4-13C3]butanesulfonate | 13C3-PFBS | 108 | 16 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]hexane sulfonate | 13C2-4:2FTS | 97 | 12 |
| Perfluoro-n-[1,2,3,4,6-13C5]hexanoic acid | 13C5-PFHxA | 115 | 14 |
| 2,3,3,3-Tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy13C3-propanoic acid | 13C3-HFPO-DA | 89 | 14 |
| Perfluoro-n-[1,2,3,4-13C4]heptanoic acid | 13C4-PFHpA | 110 | 14 |
| Sodium perfluoro-1-[1,2,3-13C3]hexanesulfonate | 13C3-PFHxS | 114 | 14 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]-octane sulfonate | 13C2-6:2FTS | 92 | 7 |
| Perfluoro-n-[13C8]octanoic acid | 13C8-PFOA | 113 | 14 |
| Sodium perfluoro-[13C8]octanesulfonate | 13C8-PFOS | 102 | 7 |
| Perfluoro-n-[13C9]nonanoic acid | 13C9-PFNA | 101 | 9 |
| Perfluoro-n-[1,2,3,4,5,6-13C6]decanoic acid | 13C6-PFDA | 111 | 12 |
| Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]-decane sulfonate | 13C2-8:2FTS | 118 | 14 |
| Perfluoro-n-[1,2,3,4,5,6,7-13C7]undecanoic acid | 13C7-PFUnA | 114 | 13 |
| Perfluoro-n-[1,2-13C2]dodecanoic acid | 13C2-PFDoA | 112 | 13 |
Conclusions
EPA Method 533 PFAS analysis in drinking water can be challenging with low-level analysis complicated by background contamination. Resprep WAX cartridges have been shown to provide performance that meets or exceeds the requirements of EPA Method 533, allowing for analysis of PFAS at ng/L levels and lower. Visit www.restek.com/PFAS for additional products, methods, and technical resources.
References
- U.S. Environmental Protection Agency, Method 533, Determination of per-and polyfluoroalkyl substances in drinking water by isotope dilution anion exchange solid phase extraction and liquid
chromatography/tandem mass spectrometry, November 2019. https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf - U.S. Environmental Protection Agency, Definition and procedure for the determination of the method detection limit, revision 2, December 2016.
https://www.epa.gov/sites/default/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf

