PFAS LC Column Anatomy: Which Phase, Dimensions, and Particle Type Are Best?
If you work in one of the many labs routinely testing samples for per- and polyfluoroalkyl substances (PFAS), you know that awareness and interest are growing as we continue to better understand the pervasiveness, persistence, and potential health risks associated with these “forever chemicals.” As interest grows, the need for fast, accurate, and precise testing is expanding with it. This demand is driving the development of better methods, and LC column selection is the foundation for building an improved approach. This becomes particularly important as the list of monitored PFAS grows to include shorter and shorter alkyl chain compounds. Here, we’ll examine the properties that are important to consider when choosing an LC column for PFAS analysis.
Column Phase & Dimension Selection
The first decision to make when determining which PFAS LC column to use is identifying an effective stationary phase. Knowing what range of PFAS you are interested in studying is crucial to this choice.
Short-Chain PFAS and Above
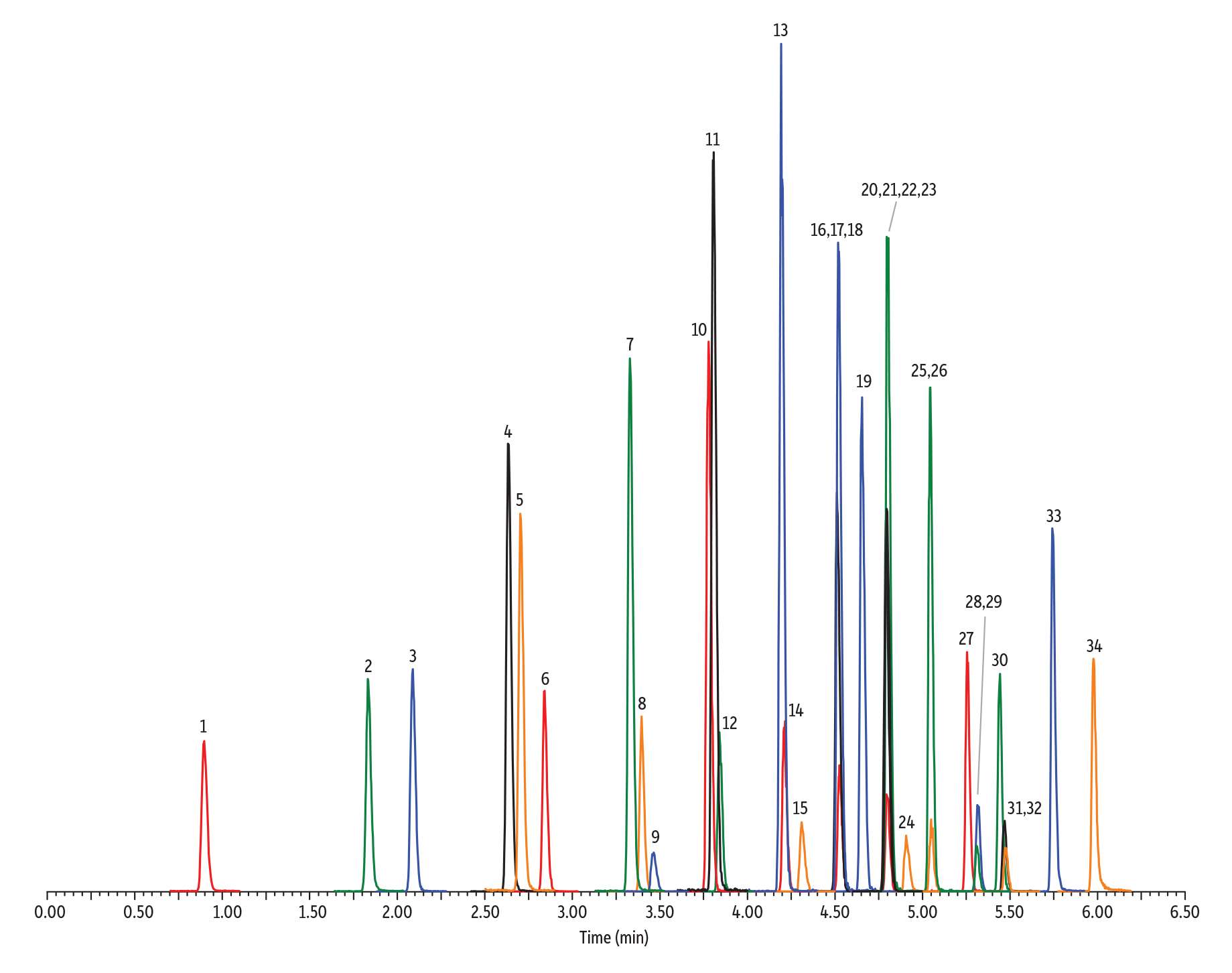
When it comes to short-chain PFAS (C4-C6) and above, our scouting of different phase chemistries showed the C18 phase was the best choice. As the alkyl chain on PFAS molecules gets longer, the interactions between those chains and the C18 ligand increase, providing a great mechanism for retention and resolution. Retention is strong enough that a relatively short and narrow column can be used to quickly and effectively resolve target analytes. The example in Figure 1 shows that a 50 x 2.1 mm Raptor C18 column easily elutes and separates the compounds of interest while meeting all of the EPA 537.1 method criteria for drinking water testing in less than 8 minutes (10 minute total analysis time).
Figure 1: A 50 x 2.1 mm Raptor C18 column is a great PFAS LC column choice; it meets all EPA 537.1 method criteria in a fast 10-minute total cycle time.

| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | Perfluorobutanesulfonic acid (PFBS) | 2.06 | 10 | 298.8 | 79.9 |
| 2. | Perfluoro-n-[1,2-13C2]hexanoic acid (13C2-PFHxA) | 3.03 | 5 | 314.9 | 270.0 |
| 3. | Perfluorohexanoic acid (PFHxA) | 3.04 | 5 | 312.9 | 268.7 |
| 4. | Tetrafluoro-2-heptafluoropropoxy-13C3-propanoic acid (13C3-HFPO-DA) | 3.36 | 5 | 286.8 | 168.7 |
| 5. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 3.38 | 5 | 285.0 | 168.9 |
| 6. | Perfluoroheptanoic acid (PFHpA) | 4.09 | 5 | 362.8 | 318.8 |
| 7. | Perfluorohexanesulfonic acid (PFHxS) | 4.22 | 10 | 398.8 | 79.9 |
| 8. | 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.24 | 5 | 376.9 | 250.7 |
| 9. | Perfluoro-[1,2-13C2]octanoic acid (13C2-PFOA) | 4.88 | 5 | 415.0 | 370.0 |
| 10. | Perfluorooctanoic acid (PFOA) | 4.90 | 5 | 413.1 | 368.9 |
| 11. | Perfluorononanoic acid (PFNA) | 5.54 | 5 | 462.9 | 418.9 |
| 12. | Perfluoro-1-[1,2,3,4-13C4]octanesulfonic acid (13C4-PFOS) | 5.57 | 10 | 503.0 | 80.0 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 13. | Perfluorooctanesulfonic acid (PFOS) | 5.58 | 10 | 498.9 | 80.0 |
| 14. | 9-Chlorohexadecafluoro-3-oxanone-1-sulfonic acid (9Cl-PF3ONS) | 5.88 | 5 | 530.8 | 350.7 |
| 15. | Perfluoro-n-[1,2-13C2]decanoic acid (13C2-PFDA) | 6.08 | 5 | 514.9 | 469.9 |
| 16. | Perfluorodecanoic acid (PFDA) | 6.08 | 5 | 512.9 | 469.0 |
| 17. | N-deuteriomethylperfluoro-1-octanesulfonamidoacetic acid (d3-NMeFOSAA) | 6.28 | 10 | 572.9 | 418.8 |
| 18. | N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) | 6.30 | 10 | 569.8 | 418.8 |
| 19. | N-deuterioethylperfluoro-1-octanesulfonamidoacetic acid (d5-NEtFOSAA) | 6.51 | 10 | 588.9 | 418.8 |
| 20. | N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) | 6.52 | 10 | 583.8 | 418.8 |
| 21. | Perfluoroundecanoic acid (PFUnA) | 6.55 | 5 | 562.9 | 518.8 |
| 22. | 11-chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 6.77 | 5 | 630.7 | 451.0 |
| 23. | Perfluorododecanoic acid (PFDoA) | 6.95 | 5 | 612.7 | 568.9 |
| 24. | Perfluorotridecanoic acid (PFTrDA) | 7.30 | 5 | 662.7 | 618.8 |
| 25. | Perfluorotetradecanoic acid (PFTA) | 7.60 | 5 | 712.7 | 668.7 |
| Column | Raptor C18 (cat.# 9304A52) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 96:4 Methanol:water | ||||||||||||||||||||
| Conc.: | 5-10 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | HPLC |
Ultrashort-Chain PFAS
When C8 PFAS were banned, other compounds with shorter alkyl chains were commercially adopted, and as the list of PFAS of interest grows, compounds with chains shorter than four carbons, or "ultrashort chain" (C2 and C3) are getting more attention. As the length of the carbon chain decreases, the influence of the polar head increases, ultimately decreasing retention on a C18 column, which has a retention mechanism based primarily on hydrophobic interaction.
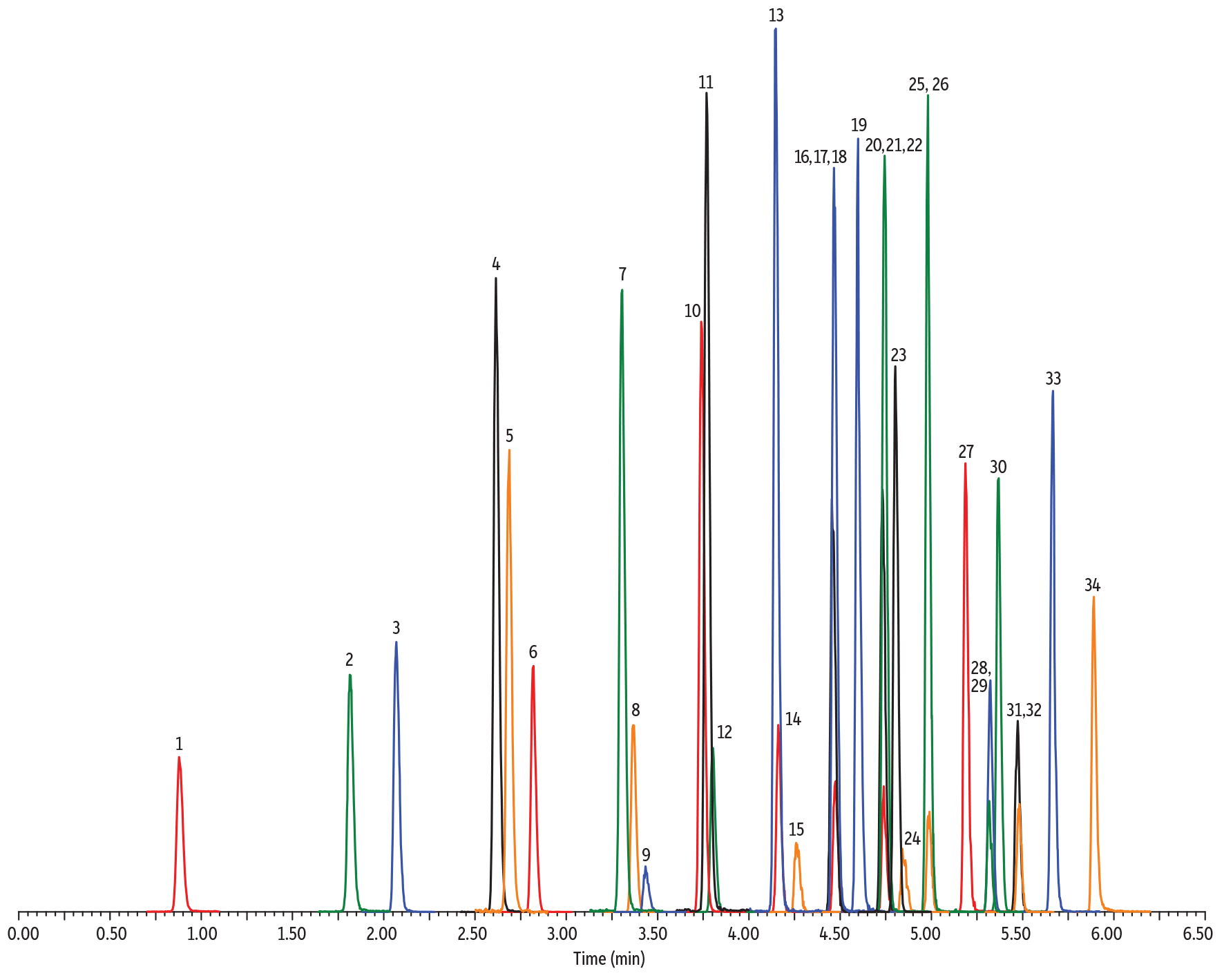
For C3 PFAS compounds like perfluoropropanoic acid (PFPrA) and perfluoropropanesulfonic acid (PFPrS), a C18 phase will still work when appropriate column dimensions are selected. In Figure 2, for example, a 100 x 3 mm Raptor C18 shows great performance, easily incorporating C3-length PFAS in a quick 11-minute analysis.
Figure 2: The Raptor C18 stationary phase will also effectively separate short-chain PFAS, but column dimensions must be increased to ensure adequate retention.
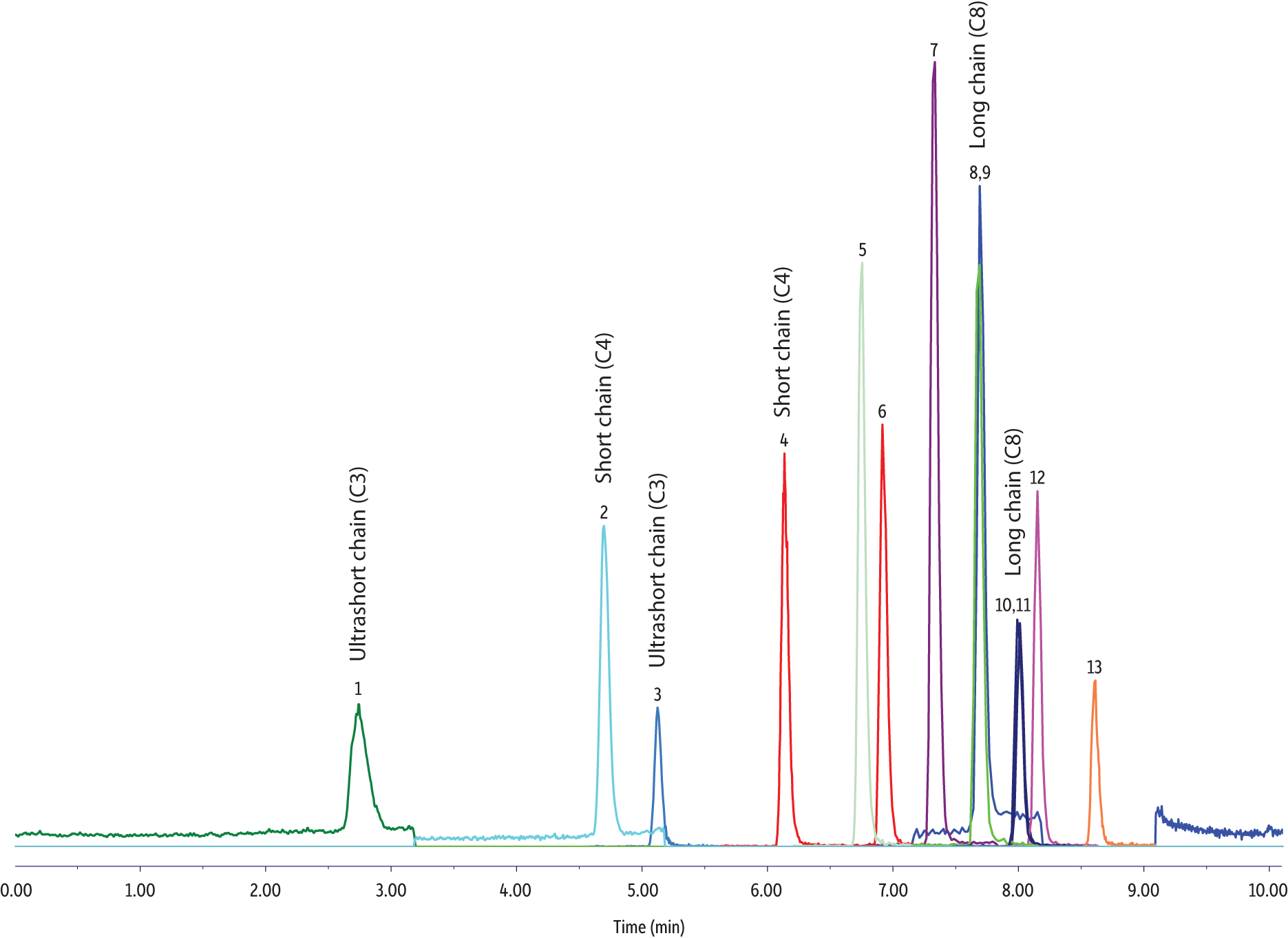
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | Perfluoropropanoic acid (PFPrA) | 2.74 | 80 | 162.9 | 119.0 |
| 2. | Perfluorobutanoic acid (PFBA) | 4.69 | 80 | 212.8 | 169.0 |
| 3. | Perfluoropropanesulfonic acid (PFPrS) | 5.13 | 80 | 248.8 | 79.6 |
| 4. | Perfluorobutanesulfonic acid (PFBS) | 6.14 | 80 | 298.8 | 79.9 |
| 5. | Perfluoro-n-[1,2-13C2]hexanoic acid (13C2-PFHxA) | 6.75 | 50 | 314.9 | 270.0 |
| 6. | Hexafluoropropylene oxide-dimer acid (HFPO-DA) | 6.92 | 80 | 285.0 | 168.9 |
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 7. | Ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) | 7.33 | 80 | 376.9 | 250.7 |
| 8. | Perfluorooctanoic acid (PFOA) | 7.70 | 80 | 413.1 | 368.9 |
| 9. | Perfluoro-[1,2-13C2]octanoic acid (13C2-PFOA) | 7.70 | 50 | 415.0 | 370.0 |
| 10. | Perfluorooctanesulfonic acid (PFOS) | 8.01 | 80 | 498.8 | 80.0 |
| 11. | Perfluoro-[1,2,3,4-13C4]octanesulfonic acid (13C4-PFOS) | 8.01 | 50 | 503.0 | 80.0 |
| 12. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS) | 8.15 | 80 | 530.8 | 350.7 |
| 13. | 11-Chloroeicosafluoro-3-oxanonane-1-sulfonate (11Cl-PF3OUdS) | 8.61 | 80 | 630.7 | 451.0 |
| Column | Raptor C18 (cat.# 9304A1E) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 3 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Conc.: | 80 ppt | ||||||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
| Sample Preparation | In a polypropylene vial, 250 µL of reagent water (fortified at 80 ppt) was mixed with 250 µL of 40:60 reagent water:methanol and 5 µL of internal standard solution (5 ng/mL of 13C2-PFHxA, 13C2-PFOA, 13C4-PFOS in methanol). The vial was capped with a polyethylene cap prior to analysis. |
| Notes | A PFAS delay column (cat.# 27854) was installed between the pump mixer and the injector. |
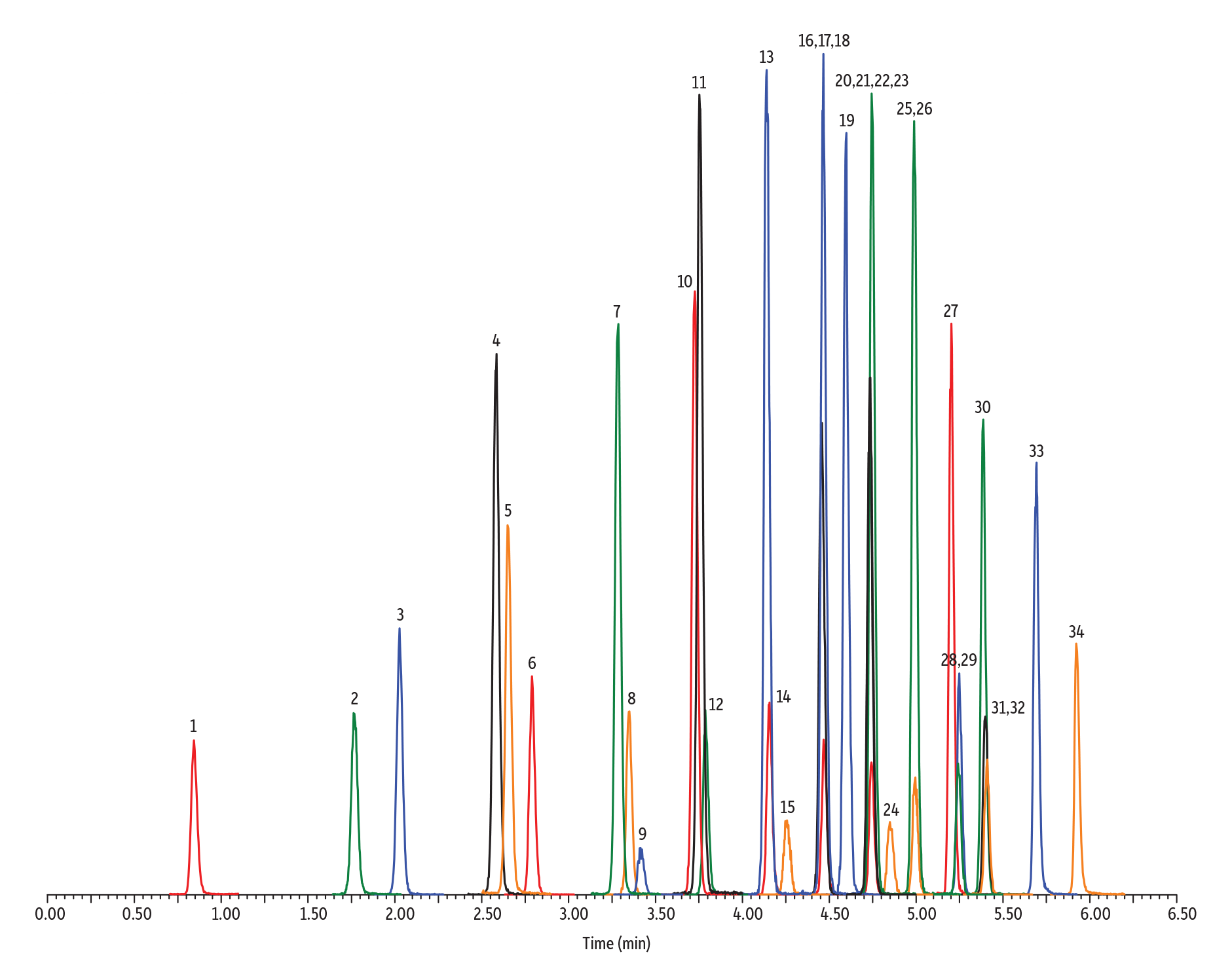
If C2 PFAS (e.g., trifluoroacetic acid) eventually make it on a list of monitored compounds, an alternate phase chemistry that targets the polar moiety of a PFAS molecule is required. Moving from the mainly hydrophobic interactions of a C18 column to a Raptor Polar X column—which has a stationary phase that is capable of both ion-exchange and HILIC separation modes—permits the retention of ultrashort-chain PFAS. Along with ultrashort-chain PFAS, the Raptor Polar X column is also capable of retaining and separating short-chain, legacy, and alternate PFAS compounds in the same analysis, providing the most comprehensive single-analysis PFAS method, as shown in Figure 3.
Figure 3: Raptor Polar X columns utilize multiple retention modes, making them the best choice for analyzing ultrashort-chain, legacy, and alternative PFAS with a single method.
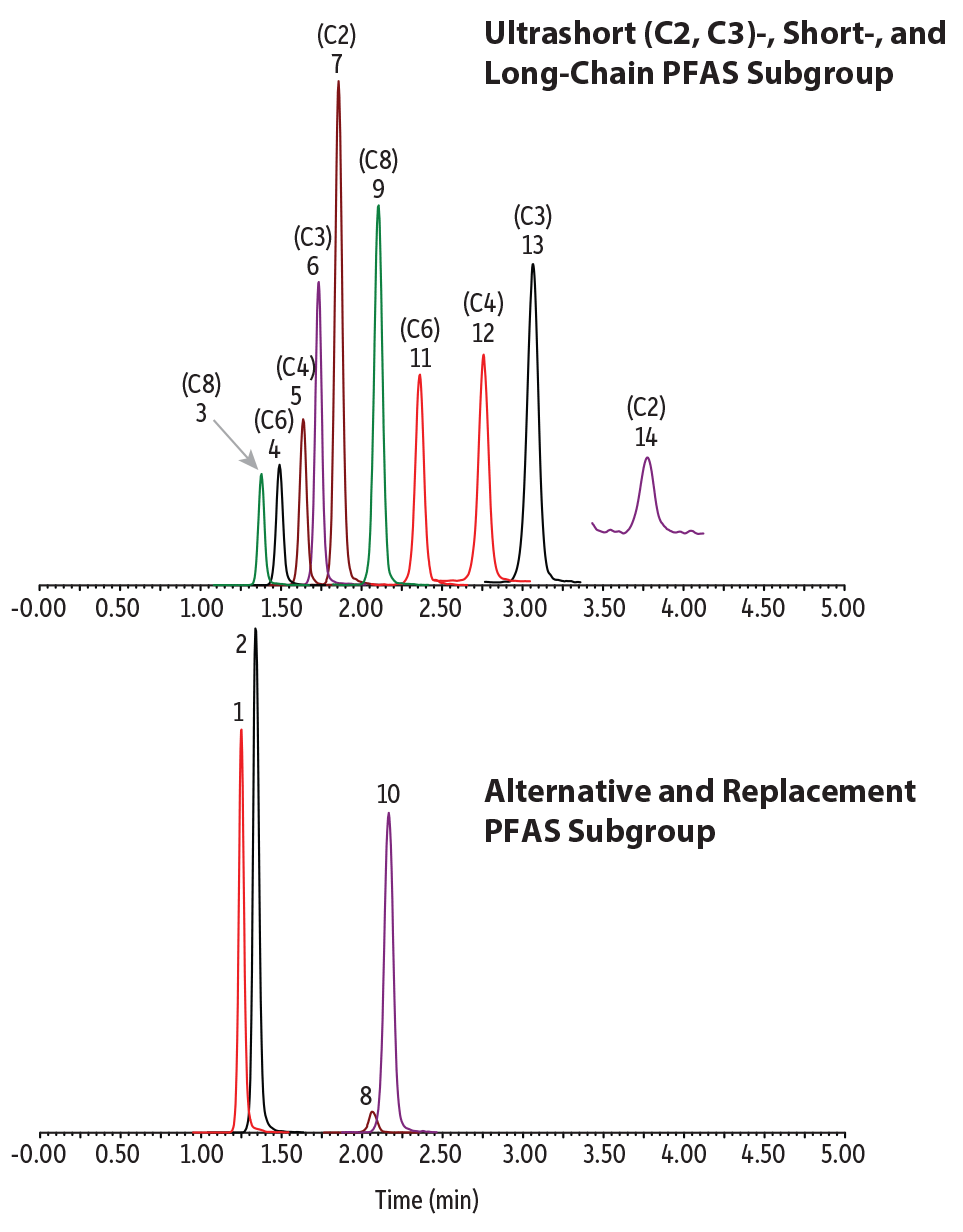
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | 11-Chloroeicosafluoro-3-oxanonane-1-sulfonate (11CL-PF3OUdS) | 1.25 | 400 | 630.78 | 450.80 |
| 2. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS) | 1.34 | 400 | 530.78 | 350.85 |
| 3. | Perfluorooctanesulfonic acid (PFOS) | 1.38 | 400 | 498.84 | 79.97 |
| 4. | Perfluorohexanesulfonic acid (PFHxS) | 1.49 | 400 | 398.90 | 79.97 |
| 5. | Perfluorobutanesulfonic acid (PFBS) | 1.64 | 400 | 298.97 | 79.97 |
| 6. | Perfluoropropanesulfonic acid (PFPrS) | 1.73 | 400 | 248.97 | 79.98 |
| 7. | Perfluoroethanesulfonic acid (PFEtS) | 1.86 | 400 | 198.98 | 79.92 |
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 8. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 2.06 | 400 | 284.97 | 168.92 |
| 9. | Perfluorooctanoic acid (PFOA) | 2.11 | 400 | 412.90 | 368.91 |
| 10. | Ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) | 2.15 | 400 | 376.90 | 250.93 |
| 11. | Perfluorohexanoic acid (PFHxA) | 2.36 | 400 | 312.97 | 268.90 |
| 12. | Perfluorobutanoic acid (PFBA) | 2.76 | 400 | 212.97 | 168.97 |
| 13. | Perfluoropropionic acid (PFPrA) | 3.06 | 400 | 163.03 | 119.01 |
| 14. | Trifluoroacetic acid (TFA) | 3.77 | 400 | 113.03 | 69.01 |
| Column | Raptor Polar X (cat.# 9311A52) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||
| Temp.: | 40 °C | ||||||||||||
| Standard/Sample | |||||||||||||
| Diluent: | 50:50 Water:methanol | ||||||||||||
| Conc.: | 400 ng/L | ||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||
| Mobile Phase | |||||||||||||
| A: | Water, 10 mM ammonium formate, 0.05% formic acid | ||||||||||||
| B: | 60:40 Acetonitrile:methanol, 0.05% formic acid | ||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
Column Particle Size and Particle Type Selection
In addition to phase chemistry and column dimensions, choices about particle size and type have to be made. Ultimately, a 2.7 µm superficially porous particle (SPP), such as those used in Raptor columns, is the most versatile choice. PFAS LC columns made with these particles produce efficient chromatography that rivals the sub-2 µm fully-porous particles (FPP) without the associated ultra-high pressure, which allows labs using either UHPLC or HPLC instruments to obtain fast, efficient analyses.
However, choice in particle size and type will have a greater or lesser effect depending on your instrument setup. For instance, labs operating traditional HPLC instruments and regularly using 5 µm FPP columns may be surprised at the improvement an SPP column can offer, regardless of the particle size. With SPP columns, you could easily see improvements in chromatographic efficiency and better peak shapes while still operating in the pressure region of most HPLC instruments. Faster runs with the same equipment are a winning combination for many labs interested in increasing sample throughput without the capital expense of a UHPLC instrument.
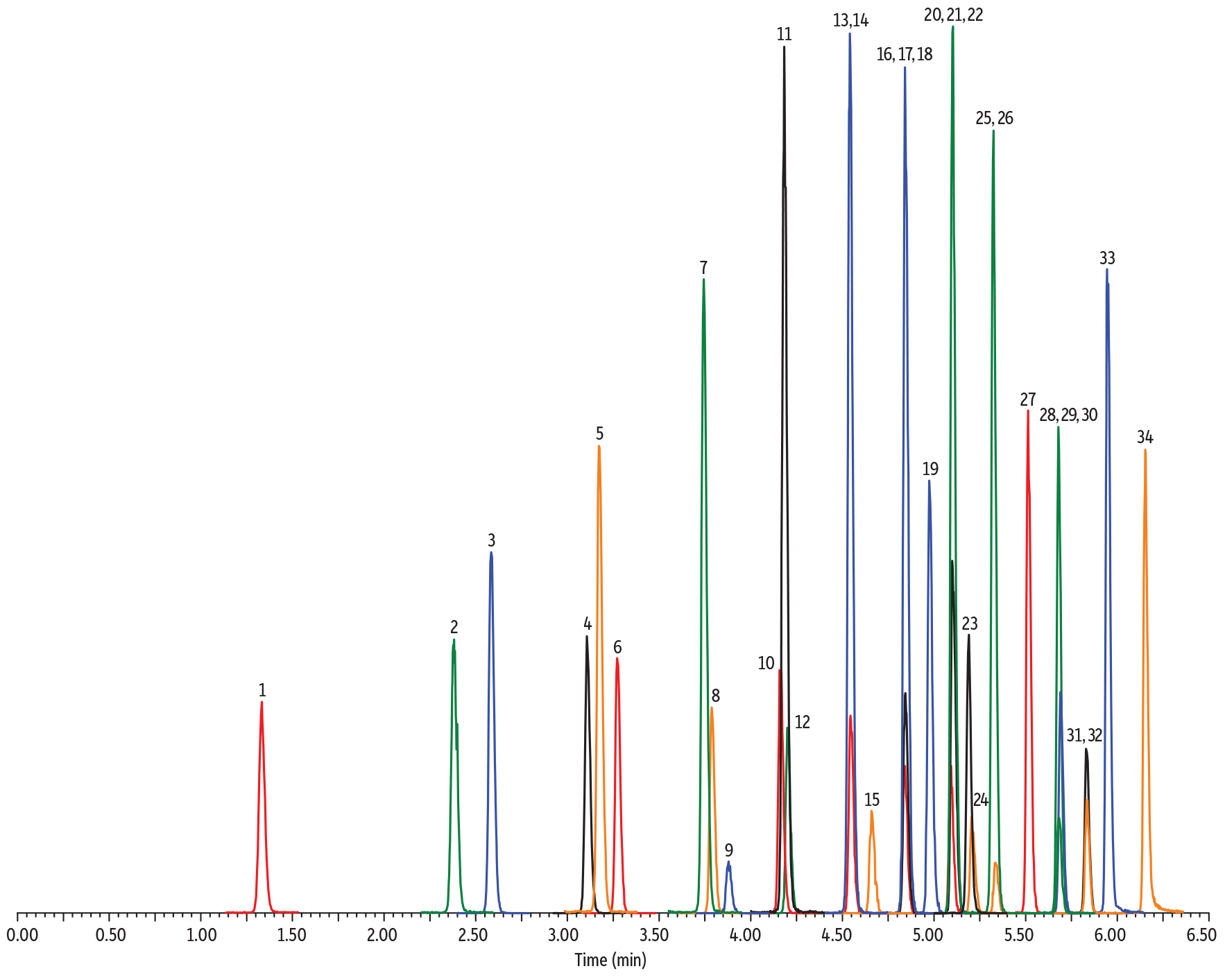
For those labs already using UHPLC instruments, the choice of particle size, especially for SPP-packed columns, makes less of a difference in efficiency and analysis speed. Figure 4 illustrates the effect of particle size using three Raptor SPP columns and a panel of PFAS on a UHPLC system. Even though the peaks are narrowest for the 1.8 µm particle column, as dictated by general chromatographic principles, the difference in the observed efficiency of 5 µm and 1.8 µm columns was not significant. However, the difference in the back pressure generated while achieving those very similar results is significant, with pressures being in the range of traditional HPLC instruments for the 5 and 2.7 µm particle columns. Using a PFAS LC column packed with these particles, you can get UHPLC performance without UHPLC pressure.
Figure 4: For SPP columns, similar chromatographic performance can be obtained with different particle sizes, but choosing 2.7 or 5 µm particle columns keeps back pressure within the limits of traditional HPLC systems. (All columns are 50 mm x 2.1 mm.)
1.8 µm Raptor C18
6500–8000 psi
2.7 µm Raptor C18
4000–5500 psi
5 µm Raptor C18
2000–3500 psi
When it comes to generating the most efficiency—which often translates into fast run times—while keeping instrument back pressure as low as possible, a PFAS LC column containing SPP particles is the clear winner. However, many labs that have a long history using FPP columns may prefer to continue using them. The good news is that an FPP column can be used to successfully analyze PFAS as well. The higher surface area and carbon load in an FPP column, such as a Force C18 column, results in increased chromatographic retention compared to an SPP column (Raptor C18) of similar particle size (Figure 5).
Figure 5: If an FPP column is preferred, Force C18 columns provide effective separations for PFAS analysis. (All columns are 50 mm x 2.1 mm.)
1.8 µm Raptor C18
6500–8000 psi
1.8 µm Force C18
7550–9250 psi
Conclusion
When choosing a PFAS LC column, a 2.7 µm Raptor SPP column will provide excellent resolution, short run times, and compatibility with both UHPLC and HPLC instruments. The choice of stationary phase depends on the range of PFAS being monitored, and a Raptor C18 column is an excellent choice for PFAS down to C3 chain lengths. However, for the most comprehensive single analysis, including the full range of ultrashort-chain (C2-C3) PFAS and above, as well as alternate PFAS, a Raptor Polar X column is the best choice.
Note that for trace-level analysis using a Raptor C18 column, false positives or elevated responses can be mitigated by adding a PFAS delay column. Use of a PFAS delay column will eliminate instrument-related background PFAS contaminants that can coelute with sample analytes.